February 4, 2021 N Engl J Med 2021; 384:481-483 DOI: 10.1056/NEJMc2033369 304 Citing Articles
TO THE EDITOR:
We conducted postmortem high-resolution magnetic resonance imaging (magnetic resonance microscopy) of the brains of patients with coronavirus disease 2019 (Covid-19) (median age, 50 years) and histopathological examination that focused on microvascular changes in the olfactory bulb and brain stem. (See the Materials and Methods section in the Supplementary Appendix, available with the full text of this letter at NEJM.org.) Images were obtained from the brains of 13 patients with the use of an 11.7-Tesla scanner at a resolution of 25 μm for the olfactory bulb and at a resolution of 100 μm for the brain. Abnormalities were seen in the brains of 10 patients. We examined the brains of patients that showed abnormalities by means of multiplex fluorescence imaging (in 5 patients) and by means of chromogenic immunostaining (in 10 patients). We performed conventional histopathological examination of the brains of 18 patients. Fourteen patients had chronic illnesses, including diabetes and hypertension, and 11 had been found dead or had died suddenly and unexpectedly. Of the 16 patients with available medical histories, 1 had delirium, 5 had mild respiratory symptoms, 4 had acute respiratory distress syndrome, 2 had pulmonary embolism, and the symptoms were not known in 3 (Table S1 in the Supplementary Appendix).Figure 1.
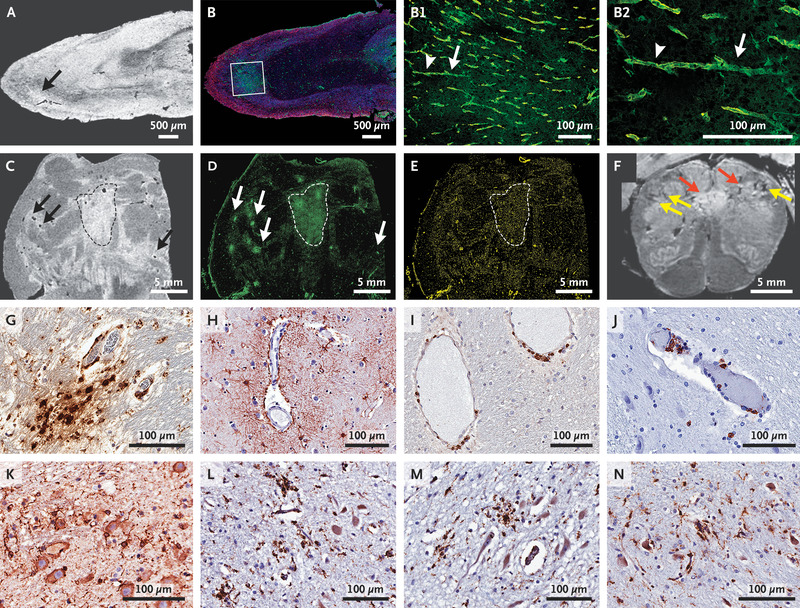
Pathological Studies of Microvascular Injury in the Brains of Patients Who Died from Covid-19.
Magnetic resonance microscopy showed punctate hyperintensities in 9 patients, which represented areas of microvascular injury and fibrinogen leakage. These features were observed on corresponding histopathological examination performed with the use of fluorescence imaging (Figure 1A and 1B). These areas showed thinning of the basal lamina of the endothelial cells, as determined by collagen IV immunostaining in 5 patients (Fig. 1B1 and 1B2). Punctate hypointensities on imaging in 10 patients corresponded to congested blood vessels (Figure 1C) with surrounding areas of fibrinogen leakage (Figure 1D and Fig. S1) and relatively intact vasculature (Figure 1E). Areas of linear hypointensities were interpreted as microhemorrhages (Figure 1F and Fig. S2). There was minimal perivascular inflammation in the specimens examined, but there was no vascular occlusion, as previously described in the Journal.1 Perivascular-activated microglia, macrophage infiltrates, and hypertrophic astrocytes were seen in 13 patients (Figure 1G and 1H, Fig. S3, and Table S4).2 There were CD3+ and CD8+ T cells in the perivascular spaces and in lumens adjacent to endothelial cells in 8 patients, which may have contributed to vascular injury (Figure 1I and 1J), as suggested in a previous report.3 Activated microglia were found adjacent to neurons in 5 patients, which is suggestive of neuronophagia in the olfactory bulb, substantia nigra, dorsal motor nucleus of the vagal nerve, and the pre-Bötzinger complex in the medulla, which is involved in the generation of spontaneous rhythmic breathing (Figure 1K through 1N and Fig. S3).
Severe acute respiratory syndrome coronavirus 2 was not detected by means of polymerase chain reaction with multiple primer sets, RNA sequencing of several areas of the brain, or RNA in situ hybridization and immunostaining (Table S5). It is possible that the virus was cleared by the time of death or that viral copy numbers were below the level of detection by our assays.
In a convenience sample of patients who had died from Covid-19, multifocal microvascular injury was observed in the brain and olfactory bulbs by means of magnetic resonance microscopy, histopathological evaluation, and immunohistochemical analysis of corresponding sections, without evidence of viral infection. These findings may inform the interpretation of changes observed on magnetic resonance imaging of punctate hyperintensities and linear hypointensities in patients with Covid-19. Because of the limited clinical information that was available, no conclusions can be drawn in relation to neurologic features of Covid-19.
Myoung-Hwa Lee, Ph.D.
National Institute of Neurological Disorders and Stroke, Bethesda, MDDaniel P. Perl, M.D.
Uniformed Services University of the Health Sciences, Bethesda, MD,Govind Nair, Ph.D., Wenxue Li, Ph.D., Dragan Maric, Ph.D., Helen Murray, Ph.D., Stephen J. Dodd, Ph.D., Alan P. Koretsky, Ph.D. National Institute of Neurological Disorders and Stroke, Bethesda, MD, Jason A. Watts, M.D., Ph.D.
Vivian Cheung, M.D. University of Michigan, Ann Arbor, MI