COVID-19 vaccines induce severe hemolysis in paroxysmal nocturnal hemoglobinuria
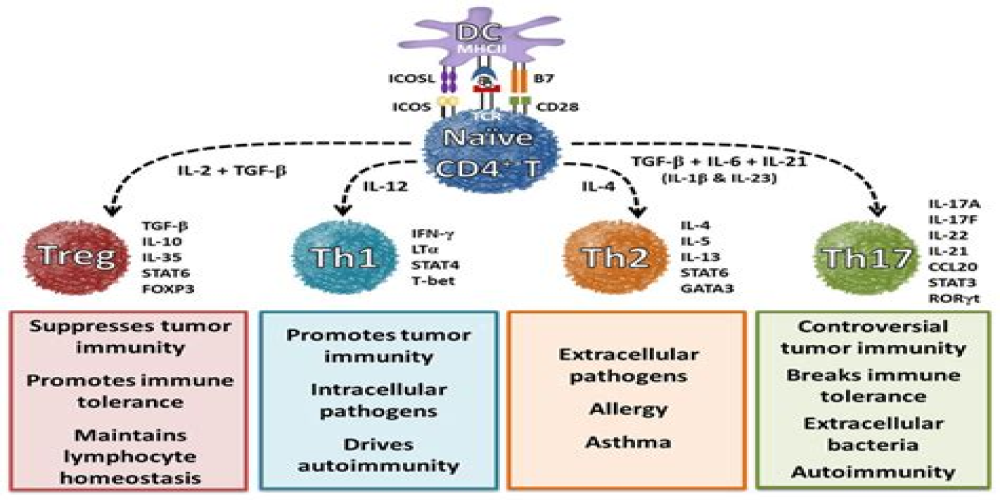
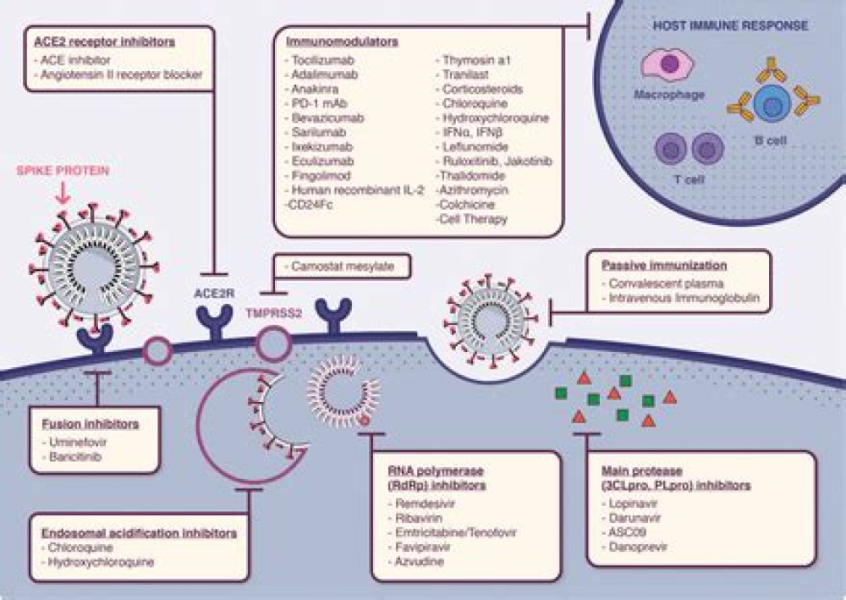
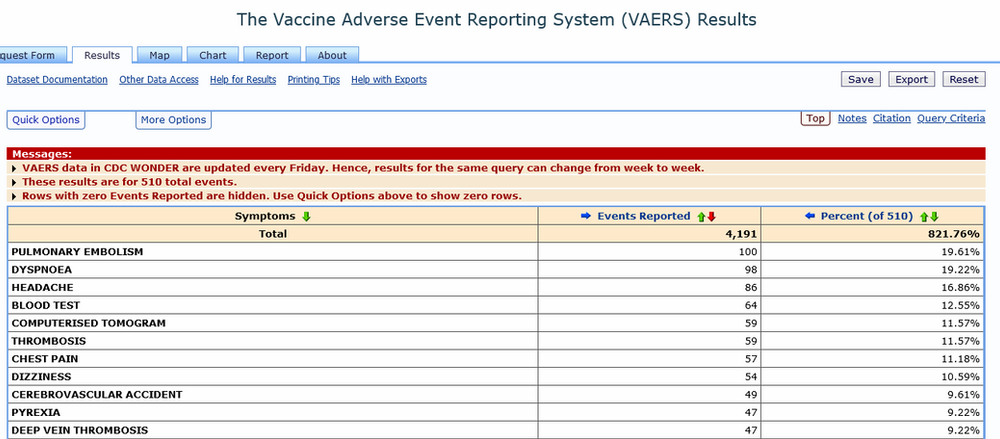
Authors: Gloria F. Gerber,1 Xuan Yuan,1 Jia Yu,1 Benjamin A.Y. Cher,2 Evan M. Braunstein,1 Shruti Chaturvedi,1 and Robert A. Brodsky1,* Complement has emerged as a likely driver of the immune response and end-organ damage in COVID-19. In patients with severe disease, deposition of terminal complement and microthrombosis have been observed in the lung, skin, kidney, and heart.1, 2, 3, 4 Recently, we demonstrated that the severe […]