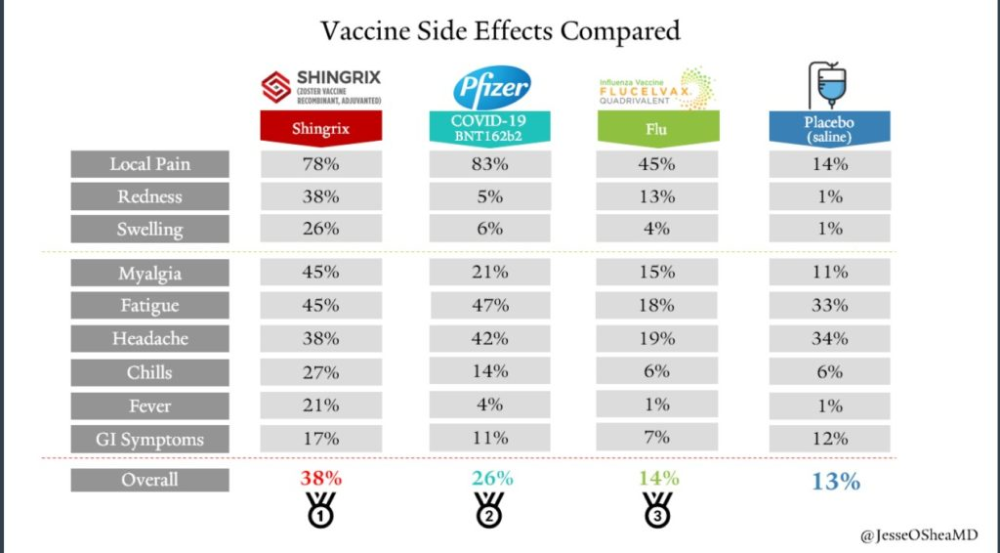
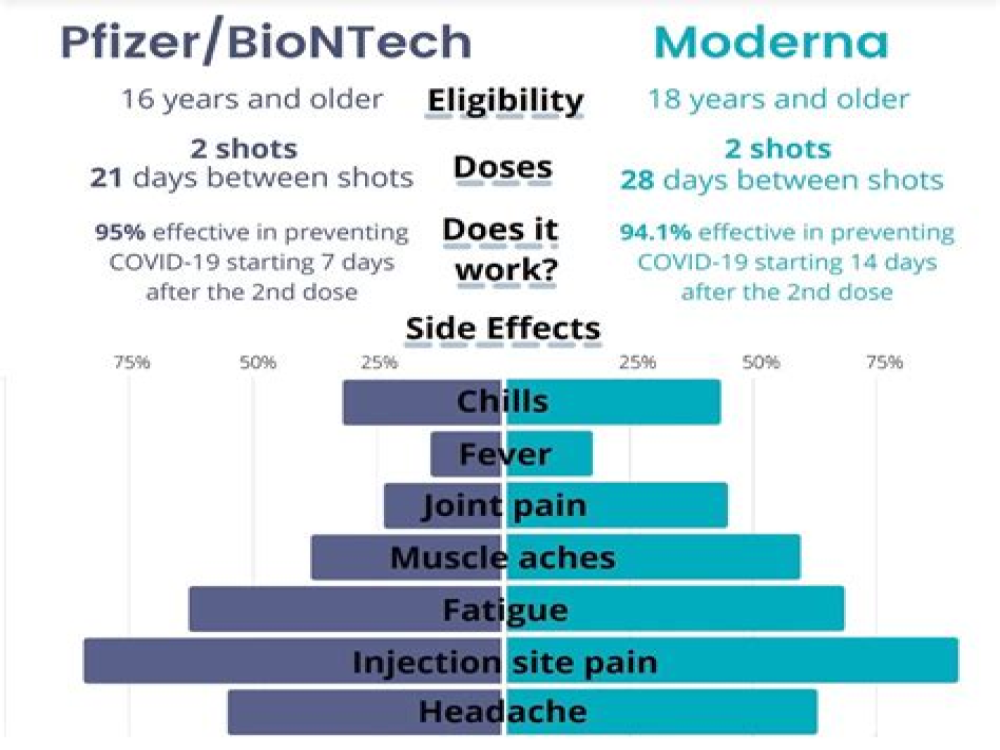
Side effects of BNT162b2 mRNA COVID-19 vaccine: A randomized, cross-sectional study with detailed self-reported symptoms from healthcare workers
Authors: Renuka A.K. Kadali,a,b,1,⁎Ravali Janagama,c,1,⁎⁎Sharanya Peruru,d and Srikrishna V. Malayalae Int J Infect Dis. 2021 May; 106: 376–381.Published online 2021 Apr 15. doi: 10.1016/j.ijid.2021.04.047 Abstract Introduction Concerns are prevailing about the safety and side effects…[...]
Read More