Authors: Williamson, Jack A. Hassard, Ecco Staller, Brian Hanley, Michael Osborn, Mauro Giacca, Andrew D. Davidson, David A. Matthews & Wendy S. Barclay
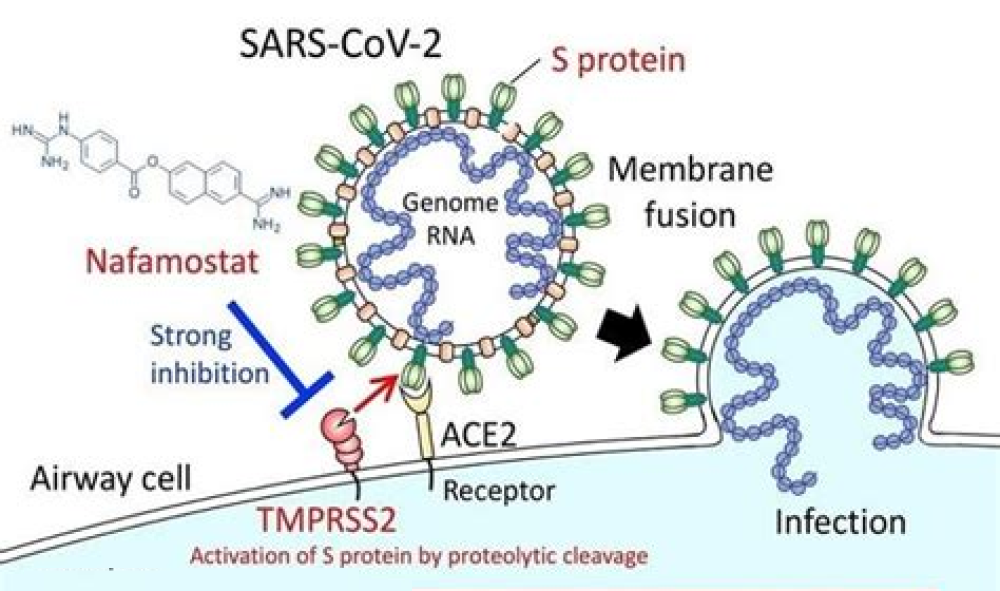
SARS-CoV-2 entry requires sequential cleavage of the spike glycoprotein at the S1/S2 and the S2ʹ cleavage sites to mediate membrane fusion. SARS-CoV-2 has a polybasic insertion (PRRAR) at the S1/S2 cleavage site that can be cleaved by furin. Using lentiviral pseudotypes and a cell-culture-adapted SARS-CoV-2 virus with an S1/S2 deletion, we show that the polybasic insertion endows SARS-CoV-2 with a selective advantage in lung cells and primary human airway epithelial cells, but impairs replication in Vero E6, a cell line used for passaging SARS-CoV-2. Using engineered spike variants and live virus competition assays and by measuring growth kinetics, we find that the selective advantage in lung and primary human airway epithelial cells depends on the expression of the cell surface protease TMPRSS2, which enables endosome-independent virus entry by a route that avoids antiviral IFITM proteins. SARS-CoV-2 virus lacking the S1/S2 furin cleavage site was shed to lower titres from infected ferrets and was not transmitted to cohoused sentinel animals, unlike wild-type virus. Analysis of 100,000 SARS-CoV-2 sequences derived from patients and 24 human postmortem tissues showed low frequencies of naturally occurring mutants that harbour deletions at the polybasic site. Taken together, our findings reveal that the furin cleavage site is an important determinant of SARS-CoV-2 transmission.
For More Information: https://www.nature.com/articles/s41564-021-00908-w#Sec