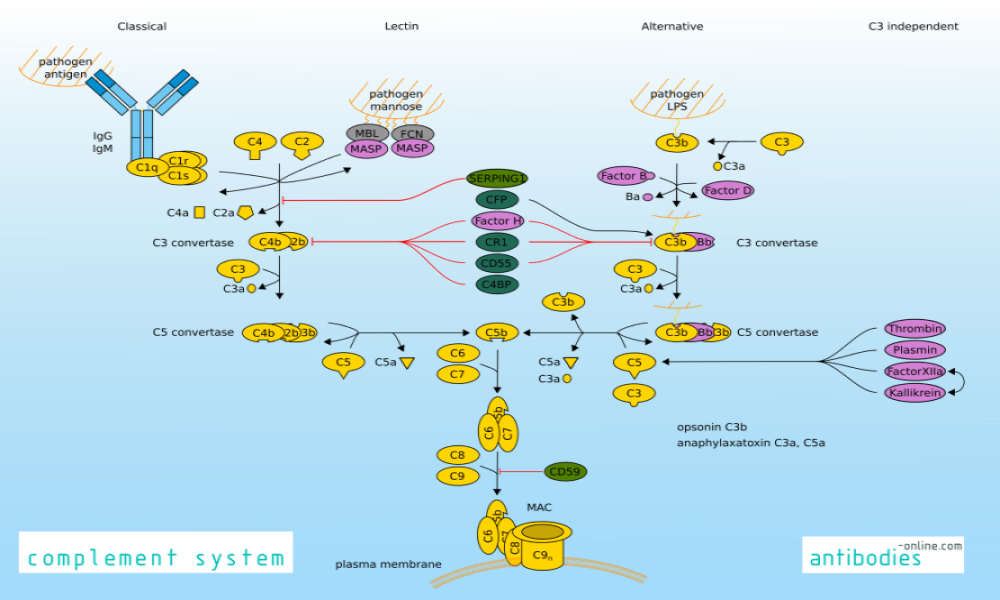
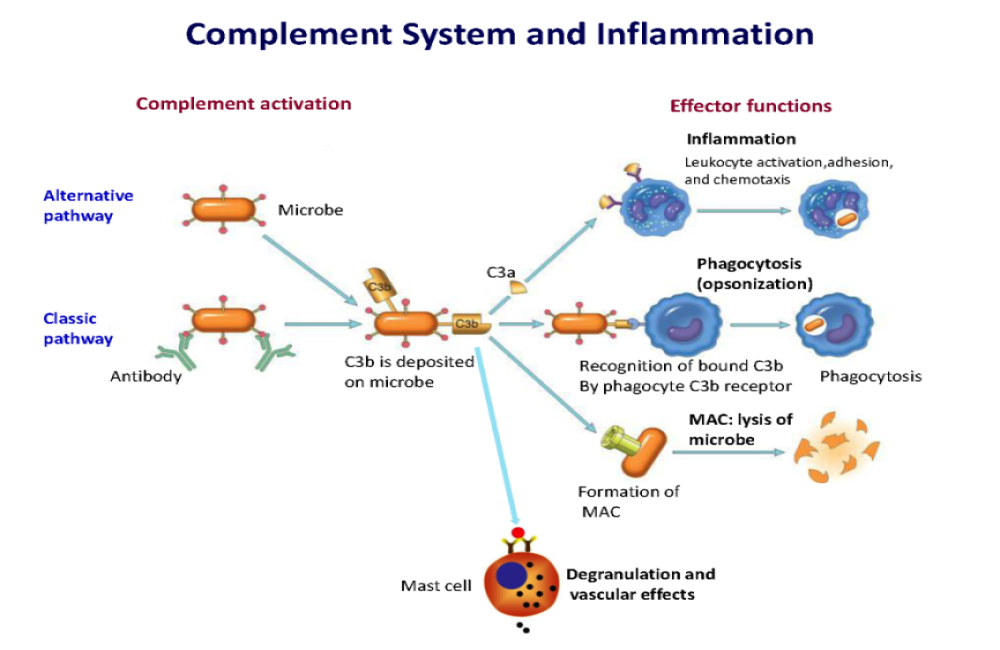
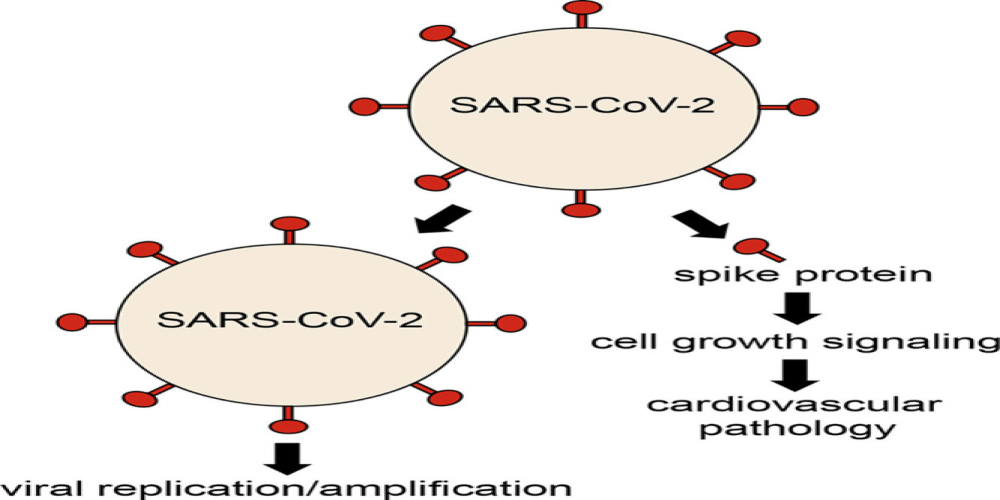
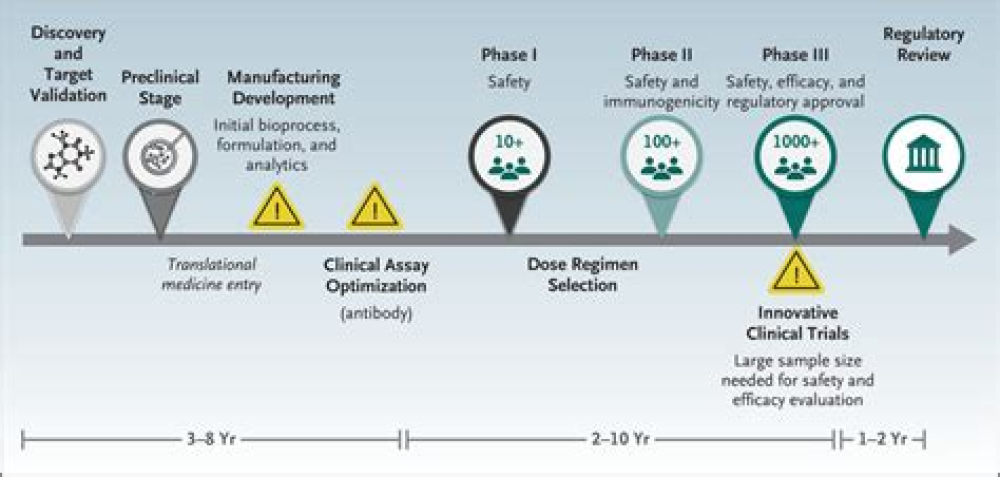
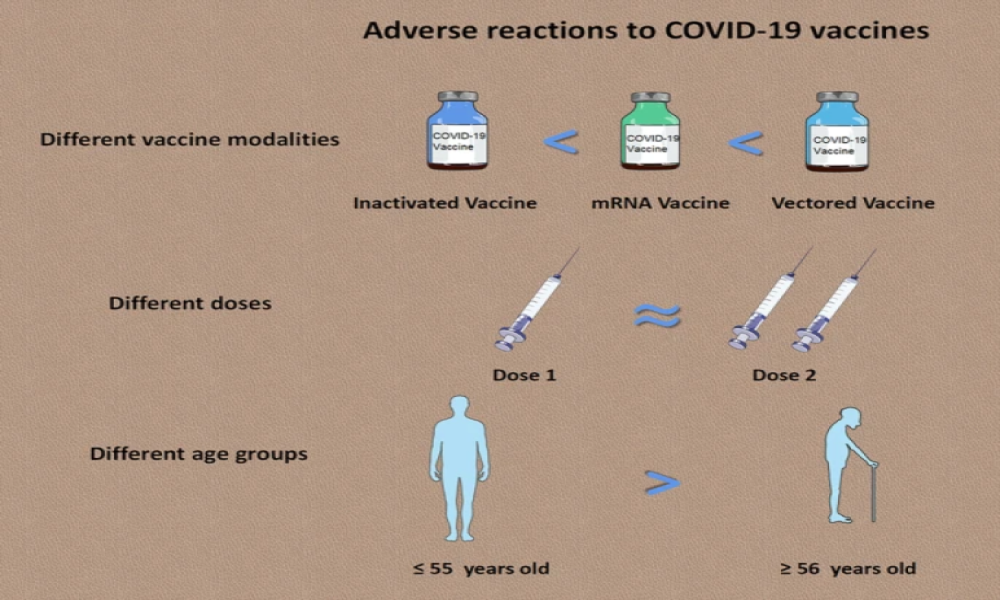
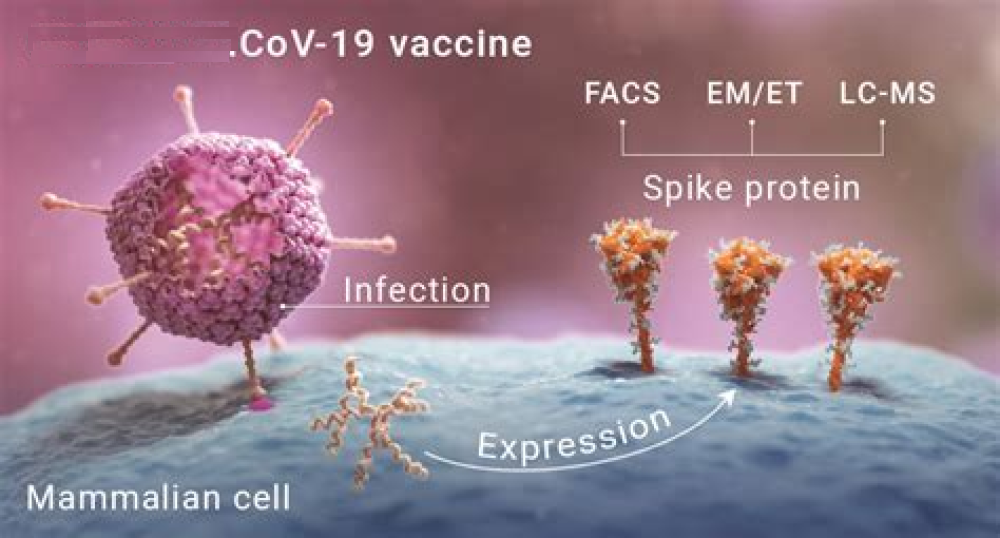
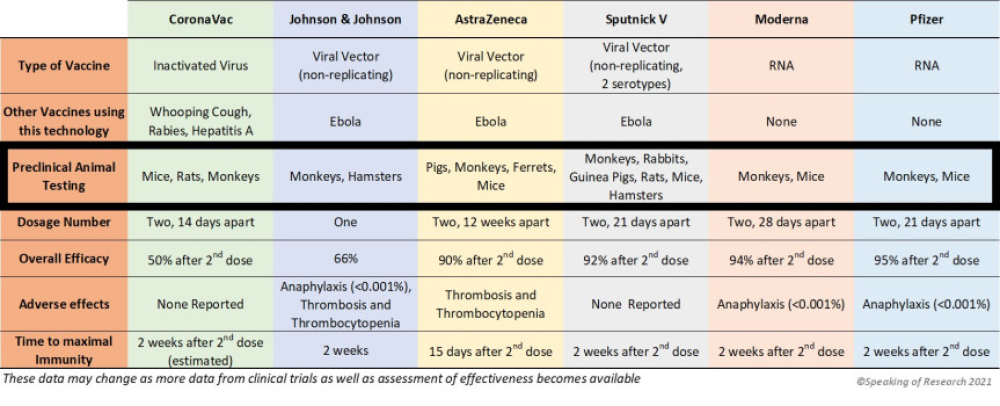
Increased complement activation is a distinctive feature of severe SARS-CoV-2 infection
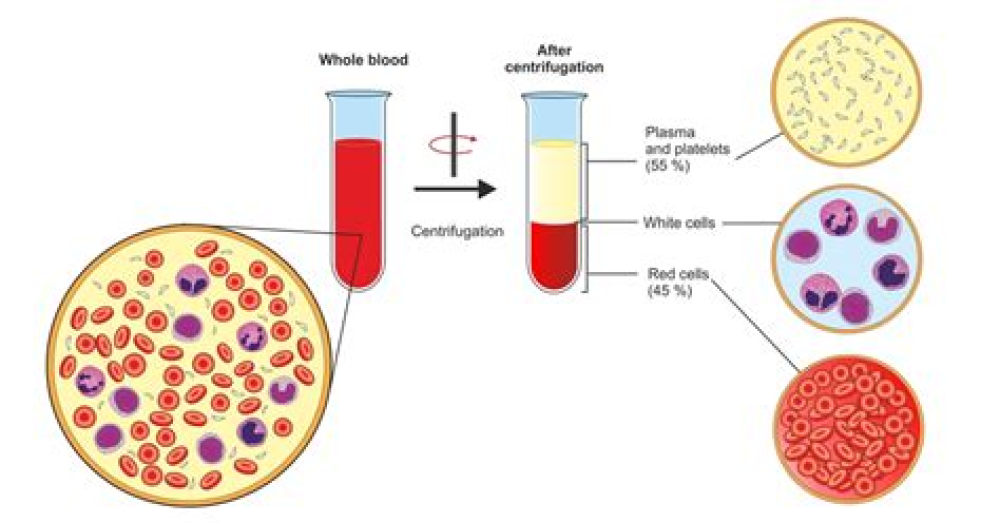
Authors: Lina Ma1, View ORCID ProfileSanjaya K. Sahu1, View ORCID ProfileMarlene Cano1, Vasanthan Kuppuswamy2, Jamal Bajwa1,3, View ORCID ProfileJa’Nia McPhatter1,4 Complement activation has been implicated in the pathogenesis of severe SARS-CoV-2 infection. However, it remains…[...]