Authors: Markus Bosmann1,2,3,4,*
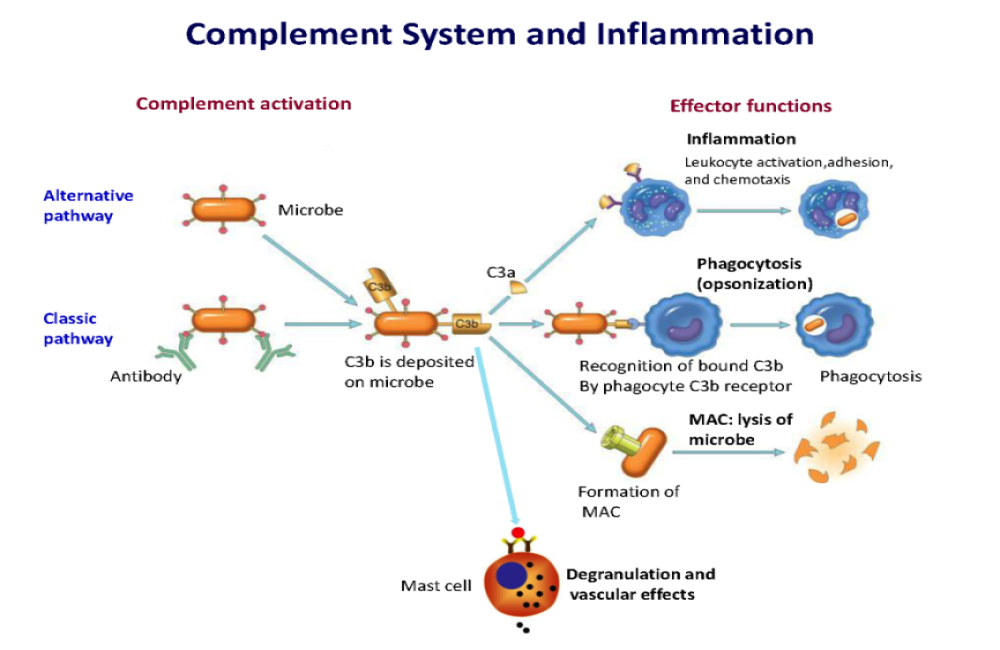
The complement system is an integral part of innate immune defense. It consists of about 50 proteins in plasma, on cell surfaces, and inside host cells. The traditional view is that complement proteins guard the local extracellular spaces and systemic bloodstream against invading pathogens. Loss-of-function mutations resulting in terminal complement pathway deficiencies are associated with a 10,000-fold higher risk for life-threatening meningococcal infections in humans. Surprisingly, the complement system is redundant for defense against most pathogens except encapsulated bacteria. Recent concepts embrace the view that complement factors mediate functions inside cells either directly or through surface receptors. Complement activity fine-tunes homeostasis, metabolism, and biogenesis. On the other hand, uncontrolled complement activation causes disease and can even worsen the outcome of infections. Toxic complement effectors mediate tissue destruction and organ injury during inflammatory diseases. Acute respiratory distress syndrome (ARDS) and sepsis are frequent and severe complications of acute infections and notorious for excessive complement consumption. The three pathways of complement activation are designed for immune sensing of nonself surfaces and foreign antigens. The mannose-binding lectin (MBL)/ficolin pathway starts with soluble pathogen pattern recognition receptors as sensors for foreign carbohydrate motifs (Fig. 1). The alternative pathway is fueled by a spontaneous “smoldering” hydrolysis of C3 targeting all surfaces, unless these surfaces present complement inhibitory proteins (CD46, CD55, and CD59) as a protective self-signal. This C3 “tick-over” is sustained by the high concentrations of C3 in plasma (1 to 2 g/liter), the highest level of all complement factors. The classical pathway is initiated by antigen-antibody complexes that are recognized by the multimeric C1 complex. As a safeguard, IgG antibodies bound in clusters or pentameric IgM are required to surpass the activation threshold. All complement pathways converge on C3 convertase complexes leading to C3 cleavage into the larger C3b and the smaller anaphylactic C3a peptides. C3b is essential for the formation of C5 convertase for cleavage of C5 into C5b and the anaphylatoxin C5a. C5b is the starting point of the pore-forming membrane attack complex (MAC) consisting of C5b-C9 with a channel diameter of ~100 Å. The C3/C5 hub represents a gigantic amplification loop. The alternative C3bBb convertase (half-life of ~3 min) cleaves additional C3, resulting in more C3bBb and so on and so forth. This enzymatic chain reaction can deposit millions of C3b molecules on target surfaces in a few seconds. It is no surprise that such explosive events need to be tightly regulated to maintain the delicate balance of effective and justified pathogen attack, while avoiding damage of innocent bystander cells.
For More Information: https://immunology.sciencemag.org/content/6/59/eabj1014.full