Authors: Dinesh Jothimani, Radhika Venugopal,∗Mohammed Forhad Abedin, Ilankumaran Kaliamoorthy, and Mohamed Rela
J Hepatol. 2020 Nov; 73(5): 1231–1240.Published online 2020 Jun 15. doi: 10.1016/j. jhep.2020.06.006PMCID: PMC7295524PMID: 32553666
Abstract
The current coronavirus disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has become a major public health crisis over the past few months. Overall case fatality rates range between 2–6%; however, the rates are higher in the elderly and those with underlying comorbidities like diabetes, hypertension and heart disease. Recent reports showed that about 2–11% of patients with COVID-19 had underlying chronic liver disease. During the previous SARS epidemic, around 60% of patients were reported to develop various degrees of liver damage. In the current pandemic, hepatic dysfunction has been seen in 14–53% of patients with COVID-19, particularly in those with severe disease. Cases of acute liver injury have been reported and are associated with higher mortality. Hepatic involvement in COVID-19 could be related to the direct cytopathic effect of the virus, an uncontrolled immune reaction, sepsis or drug-induced liver injury. The postulated mechanism of viral entry is through the host angiotensin-converting enzyme 2 (ACE2) receptors that are abundantly present in type 2 alveolar cells. Interestingly, ACE2 receptors are expressed in the gastrointestinal tract, vascular endothelium and cholangiocytes of the liver. The effects of COVID-19 on underlying chronic liver disease require detailed evaluation and, with data currently lacking, further research is warranted in this area.
Introduction
Coronaviruses are enveloped single-stranded RNA viruses, belonging to the Coronaviridae family and Orthocoronavirinae subfamily. They are some of the largest viruses (with sizes ranging from 27–34 kilobases). Coronavirus infections are commonly seen in mammals and birds. They cause zoonotic, predominantly upper respiratory tract, infections in humans. Electron microscopic images shows a ‘halo’ or ‘crown’ around the virus which explains their name. Two coronaviruses, severe acute respiratory syndrome coronavirus (SARS-CoV) and the middle eastern respiratory syndrome coronavirus (MERS-CoV), caused relatively recent epidemics, in 2003 and 2012, respectively.
The current coronavirus, responsible for the coronavirus disease 2019 (COVID-19) pandemic, has been labelled severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by the International Taxonomy group. Genome sequencing analysis showed SARS-CoV-2 is possibly a chimeric variant of a bat coronavirus identified in 2015 by Benvenuto and Colleagues.1 The resulting disease was termed COVID-19 by the World Health Organization (WHO) on the 11th February 2020. Viral detection studies by Zhou and colleagues2 showed an 80% homology between SARS-CoV (2003 pandemic) and the current novel coronavirus.
During the previous SARS epidemic, around 60% of patients developed various degrees of liver damage. Based on phylogenetic resemblance it is possible that SARS-CoV-2 also causes liver injury.
Epidemiology
Several cases of severe unexplained pneumonia were reported in Wuhan, China in December 2019. Bronchoalveolar lavage from an index case identified the presence of SARS-CoV-2 on the 3rd January 20203 and subsequently the WHO declared an ‘epidemic’. Following the rapid increase in COVID-19 infections across the world, the WHO declared a ‘pandemic’ on the 11th March 2020, an emergency public health situation. Wuhan was the initial epicentre for COVID-19, where the first 41 cases of severe pneumonia were reported following exposure to bats and pangolins at the Huanan Seafood Wholesale market.4 Subsequent cases were reported from the same locality by Chen and colleagues.5 However, several patients in the outbreak did not have exposure to animals, likely indicating person to person transmission.
Key point
COVID-19 is a pandemic caused by SARS-CoV-2, a virus that has 80% homology with SARS-CoV.
A WHO report from the 19th May 2020, confirmed 4,731,458 COVID-19 positive cases from 213 countries worldwide, of which 1,477,516 cases were reported in the United States of America, 231,606 cases in Spain, 225,886 cases in Italy, 246,410 in the United Kingdom, 84,500 cases in China (the origin of the pandemic) and 101,139 cases in India.6 These data indicate the rapid spread of the disease around the world, with a doubling rate of 7.2 days.
ACE2 receptors
As with SARS-CoV, angiotensin-converting enzyme 2 (ACE2) appears to be the susceptible receptor for SARS-CoV-2 and is expressed in more than 80% of alveolar cells in the lungs. In vitro studies from the SARS epidemic identified ACE2 as the host receptor for viral entry.7 Immunohistochemical studies from human tissues during the SARS pandemic showed high expression of the ACE2 receptor protein in the vascular endothelium of small and large arteries and veins. In the lungs, ACE2 is highly expressed in type 2 alveolar cells. Interestingly, fibrotic lungs had much higher staining for ACE2, whereas bronchial epithelial cells showed weaker expression. A recent study showed that SARS-CoV-2 possessed 10-20-fold higher receptor binding affinity.8 Immunohistochemical studies identified higher expression of ACE2 receptors in the gastrointestinal tract. ACE2 expression is high in the basal layer of the squamous epithelium. of the nasal, oral and nasopharyngeal mucosa. Smooth muscles of the gastric and intestinal colonic mucosa also express ACE2. In addition, ACE2 is abundantly expressed in enterocytes in the duodenum, jejunum and ileum.9
Key point
ACE2 is the host cell receptor for SARS-CoV-2; it is present in type 2 alveolar cells, the gastrointestinal tract and the liver.
Hepatic distribution of ACE2 is peculiar. It is highly expressed in the endothelial layer of small blood vessels, but not in the sinusoidal endothelium. Chai and colleagues10 found that the ACE2 cell surface receptor was more highly expressed in cholangiocytes (59.7%) than hepatocytes (2.6%). The level of ACE2 expression in cholangiocytes was similar to that in type 2 alveolar cells of the lungs, indicating that the liver could be a potential target for SARS-CoV-2. Immunohistochemistry stains for ACE2 were negative on Kupffer cells, as well as T and B lymphocytes.
A recent study from Wuhan showed that Asian men had higher expression of ACE2, indicating the possibility of a higher susceptibility to COVID-19 in this population.11 , 12
Transmission
SARS-CoV-2 started as a zoonotic infection; however, the disease spreads rapidly from person to person through coughing and sneezing, particularly amongst close contacts. SARS-CoV-2 is resilient and can remain viable for 2 hours to 14 days depending on the fomite and the weather condition.13
The transmission potential of an infection in the community is based on its basic reproduction rate which is usually denoted as disease transmission ratio (R0). This represents the number of secondary cases resulting from an index case in a susceptible population. The (R0 – R naught) of COVID-19 is 2.2.14
Previous studies showed that 19.6% to 73% of patients with SARS presented with gastrointestinal symptoms.[15], [16], [17], [18] Active replication of SARS-CoV was detected in the enterocytes of the small intestine.15 Moreover, SARS-CoV RNA was detected in patient stool samples during the SARS pandemic, which highlighted the possibility of faeco-oral transmission. A similar pattern has been observed with SARS-CoV-2; between 3% and 79% of patients with COVID-19 develop gastrointestinal symptoms, predominantly nausea, vomiting and diarrhoea. Zhang et al. found that 53.3% and 26.7% of oral and anal swabs remained positive for SARS-CoV-2 RNA, respectively, for several days after treatment. The same study group performed paired samples on a different cohort of patients with COVID-19 and found that on day 0, 80% of patients were positive on oral swabs whereas on day 5, 75% of patients were positive on anal swabs, indicating the dynamic changes in viral tests during the course of the illness.19 Xiao and colleagues20 showed that patients with SARS-CoV-2-related respiratory illness can continue to shed the virus in stool even after a negative respiratory sample. In a series of 73 patients with COVID-19, about 53.42% had detectable RNA in their stool, of whom about 23.29% continued to have positive RT-PCR for SARS-CoV-2 RNA in faecal samples even after a negative respiratory sample.20 Yeo and colleagues21 showed that faecal shedding can continue to occur for a longer period after clinical recovery and these patients can potentially infect others. These findings illustrate the multiple routes of viral entry into a single host, viral persistence in various organ systems and possible faecal-oral transmission of SARS-CoV-2 even during the convalescence period.
Key point
In addition to droplets, SARS-CoV-2 also transmits through the faeco-oral route.
With limited therapeutic options, prevention by social distancing appears to be the cornerstone of COVID-19 management. Virus transmission can be reduced by various methods described in the WHO protocol.6 These include, maintaining safe social distance, regular hand washing for 20 seconds, using 60% alcohol hand rub, and avoiding crowded places and public events. Countries have taken different measures to reduce viral transmission and most countries have gone into ‘Lockdown’ in order to stop viral transmission. Being a large virus particle, a surgical face mask should provide adequate protection against viral inhalation. N-95 masks should be reserved for treating teams. Personal protective equipment should be worn according to institutional policy. All patients with a history of travel to affected regions should be screened for SARS-CoV-2 even if they are asymptomatic. People with high temperature, dry cough, profound tiredness, diarrhoea or other unusual symptoms with recent travel history should be tested for COVID-19. Nations will need to continually monitor their prevention, testing and treatment strategies based on guidelines issued by the WHO.
Clinical features
Initial reports from China showed that the incubation period of SARS-CoV-2 was between 3 to 7 days and occasionally 2 weeks. The longest incubation period identified was 12.5 days.14
Large studies from a Chinese population reported fever (≥38°C), dry cough, fatigue, myalgia, leukopenia and raised liver enzymes as the most common clinical features of COVID-19 on presentation, as shown in Table 1 and and2 .2 . Nausea, vomiting and diarrhoea were seen in 2–10% of patients with COVID-19.
Table 1
Spectrum of clinical manifestations and their frequency from recent studies on COVID-19 in China.
| Clinical features | Wang et al.22 n = 138 | Zhou et al.51 n = 191 | Guan et al.23 n = 1,099 |
|---|---|---|---|
| Fever | 98.6% | 94% | 88.7% |
| Cough | 59.4% | 79% | 67.8% |
| Sputum | n.a. | 23% | 33.7% |
| Myalgia | n.a. | 15% | 14.9% |
| Fatigue | 69.6% | 23% | 38.1% |
| Diarrhoea | n.a. | 5% | 3.8% |
| Nausea/vomiting | n.a. | 4% | 5.0% |
| Sore throat | n.a. | n.a. | 13.9% |
| Lymphopenia (<0.8 × 109/L) | 70.3% | 40% | n.a. |
| Prolonged PT (>13.5 seconds) | 58% | n.a. | n.a. |
| Raised LDH (>261 U/L) | 39.9% | n.a. | n.a. |
COVID-19, coronavirus disease 2019; LDH, lactate dehydrogenase; PT, prothrombin time; n.a., data not available.
Table 2
Classification of COVID-19 into 3 groups based on severity of clinical manifestations by Chinese Center for Disease Control.23
| Mild disease (reported in 81% cases) | Fever, dry cough, mild dyspnoea (respiratory rate <30/min). |
| Severe disease (reported in 14% cases) | Dyspnoea, respiratory rate >30 and/or lung infiltrates >50% within 24 to 48 hours. |
| Critical disease (reported in 5% cases) | Respiratory failure, septic shock and/or multiple organ dysfunction or failure. |
COVID-19, coronavirus disease 2019.
In the latest case series from Wuhan by Wang and colleagues,22 138 hospitalised patients (including 40 healthcare workers and 17 already hospitalised for other conditions) with COVID-19; median age was 56 years (IQR 22–92 years) and 54.3% were males. Clinical features were fever (98.6%), fatigue (69.6%), dry cough (59.4%), lymphopenia <0.8 × 109/L (70.3%), prolonged prothrombin time (58%), and raised lactate dehydrogenase (LDH) 261 U/L (39.9%). Thirty-six patients (26.1%) received intensive care unit (ICU) care for acute respiratory distress syndrome (ARDS) (61.1%), cardiac arrhythmias (44.4%) and shock (30.6%). Onset and the progression of symptoms were dramatic, with a median time from symptoms to ARDS of only 8 days. Patients requiring intensive care were older (66 vs. 51, years) and more often had comorbidities (72% vs. 32%). Patients admitted to the ICU had higher LDH (435 U/L vs. 212 U/L, p <0.001), aspartate aminotransferase (AST) (52 U/L vs. 29 U/L, p <0.001) and hypersensitive cardiac troponin (11 ng/ml vs. 5.1 ng/ml, p = 0.004). All 138 patients showed bilateral pneumonia in the thoracic scan. Analysis between the survivors and non-survivors showed higher white blood cell count with severe progressive lymphopenia in the non-survivors. With disease progression, these patients required organ support with progressive deterioration in renal function before death.
In the largest database analysis of 1,099 patients with confirmed COVID-19 from China, by Guan and colleagues,23 the median age of presentation was 47 years (IQR 35–58 years) and 58% were male. The most common presenting symptoms were fever (88.7%), cough (67.8%), nausea or vomiting (5%), and diarrhoea (3.8%). CT chest radiography revealed ground glass opacity (56.4%) and bilateral patchy shadows (51.8%). Of 1,099 patients, 5% were admitted to the ICU, 2.3% underwent invasive ventilation and 1.4% died. COVID-19 disease was classified according to the clinical severity into 3 groups by the Chinese CDC by Guan and colleagues23 as shown in Table 2.
Key point
2–11% of patients with COVID-19 have been reported to have underlying chronic liver disease.Go to:
COVID-19 and hepatic dysfunction
It is intriguing to know the pattern of liver injury in COVID-19. Hepatic involvement in COVID-19 could be related to the direct cytopathic effect of the virus, an uncontrolled immune reaction, sepsis or drug-induced liver injury. Given the higher expression of ACE2 receptors in cholangiocytes, the liver is a potential target for SARS-CoV-2. Moreover, COVID-19 may cause worsening of underlying chronic liver disease, leading to hepatic decompensation and acute-on-chronic liver failure, with higher mortality.
A summary of recently published studies is provided in Table 3 . Overall, 2–11% of patients with COVID-19 were reported to have underlying chronic liver disease and 14-53% with COVID-19 developed hepatic dysfunction,24 particularly those with severe COVID-19. Hepatic dysfunction was significantly higher in critically ill patients and was associated with poor outcome.
Table 3
Studies of COVID-19 and hepatic manifestations.
| Author | Country | Comments |
|---|---|---|
| Chen et al.26 | China | Higher ALT and AST in deceased patients. High mortality in patients with acute liver injury (76.9%). |
| Li et al.74 | China | 7% of patients with COVID-19 had underlying chronic liver disease. |
| Wang et al.22 | China | 3.9% of patients with COVID-19 had underlying chronic liver disease. Mortality 4.3%. |
| Guan et al.23 | China | 2.1% of patients with COVID-19 had chronic hepatitis B infection. Mortality 1.4%. |
| Huang et al.4 | China | Mortality 15%. 1 (4%) patient with COVID-19 had underlying chronic liver disease. |
| Fan et al.28 | China | Patients with abnormal LFT had longer hospital stay (16.4 vs. 12.6 days). |
| Cai et al.75 | China | Higher AST, ALT and GGT in patients with severe disease. Patients with NAFLD had severe disease. |
| Cao W.76 | China | Higher ALT and AST in patients with severe COVID-19. |
| Shi et al.77 | China | 7 (3%) patients with COVID-19 had underlying chronic liver disease. |
| Wu et al.78 | China | 3% (7) had underlying CLD. Bilirubin was significantly higher in patients with ARDS-related death. |
| Graselli et al.79 | Italy | 15-30% mortality in patients between 50–70 years of age. |
| Arentz et al.80 | USA | 3 (14.7%) patients developed acute liver injury. |
| Zhang et al.24 | China | Mortality 1.7%. |
ALD, alcohol-related liver disease; ALT, alanine aminotransferase; ARDS, acute respiratory distress syndrome; AST, aspartate aminotransferase; CLD, chronic liver disease; COVID-19, coronavirus disease 2019; GGT, gamma glutamyltransferase; ICU, intensive care unit; LFT, liver function test; NAFLD, non-alcoholic fatty liver disease.
In the recent series from Wuhan, by Wang and colleagues,22 4 patients (2.9%) with COVID-19 had underlying chronic liver disease. Another study from China23 showed that 23 (2.1%) patients were positive for HBsAg, of whom only one had severe COVID-19. Interestingly, a study from outside Wuhan by Xu and colleagues25 identified 26 patients with COVID-19 in whom 11% had underlying chronic liver disease. In another study, comparing 113 non-survivors and 161 survivors showed that 4% had underlying chronic hepatitis B.26 Cases of acute liver injury were reported in 13 (5%) out of 274 patients of whom 10 (76.9%) died.26
With the current evidence, it is clear that elevated liver enzymes are observed predominantly in severe and critical cases of COVID-19. Raised AST was noted in 8/13 (62%) patients in ICU compared to 7/28 (25%) in the non-ICU setting.24 The peak alanine aminotransferase (ALT) and AST levels noted were 7,590 U/L and 1,445 U/L, respectively, in severe COVID-19.27 Interestingly, a higher proportion of enzyme elevation was noted in patients receiving lopinavir/ritonavir therapy (56.1% vs. 25%).28 It was unclear whether the elevated liver enzymes were due to the disease per se or drug-induced liver injury in this population. There is a possible effect of liver damage due to inflammatory cytokine storm in severe COVID-19.29
Key point
14–53% of patients with COVID-19 have been reported to develop some form of hepatic dysfunction.
Interestingly, despite the presence of ACE2 in cholangiocytes, more patients developed raised transaminases. An, unpublished data from Wuhan, China, by Xu et al. showed increased gamma glutamyltransferase (GGT) levels in severe cases of COVID-19.30 Whether COVID-19 aggravates cholestasis in patients with primary biliary cholangitis and primary sclerosing cholangitis requires further analysis.31 It is possible that hepatic dysfunction may result from cytokine storm rather than the direct cytopathic effects of the virus. More data is required to ascertain the pattern and the degree of liver injury in patients with COVID-19.
COVID-19 liver histology
Xu et al. reported the first post-mortem findings of a patient who succumbed to severe COVID-19. In his study, the liver histology revealed moderate microvesicular steatosis and mild inflammatory infiltrates in the hepatic lobule and portal tract. However, at this stage, it is unclear whether these changes are related to the viral infection or to the drugs. In addition, peripheral blood examination showed significantly reduced but hyper-reactive CD4 and CD8 cells in a proinflammatory state, with increased CCR6+ Th17 CD4 T cells and cytotoxicity granulations in CD8 cells, which may also contribute to hepatocellular dysfunction.32
In another report by Tian S et al., post-mortem liver biopsies in 4 patients with COVID-19 showed mild sinusoidal dilatation and focal macrovesicular steatosis. There was mild lobular lymphocytic infiltration, which was not significant in portal areas. SARS-CoV-2 RNA was isolated from liver tissue through RT-PCR in one of the patients. Though the bile duct epithelium expresses higher levels of ACE2 receptors, there was not much evidence to point towards bile duct damage.33
During the SARS-CoV outbreak in 2002, 23% to 60% of patients had hepatic dysfunction and few patients underwent liver biopsy. This revealed mild to moderate lobular lymphocytic inflammation, ballooning of hepatocytes and apoptosis. The most prominent feature was high mitotic figures indicative of a rapidly proliferative state (positive Ki-67). The Ki proliferative index of hepatocytes in chronic hepatitis C infection is around 0.45 to 1% suggestive of high replicative phase of hepatocytes in chronic hepatitis C infection. Immunohistochemistry studies showed that the Ki proliferative index of hepatocytes during SARS-CoV infection was much higher than during chronic hepatitis C infection and liver regeneration. The mitotic index was probably due to cell cycle arrest following SARS-CoV infection. It is possible that COVID-19 has a similar pathogenesis.34
Liver abnormality in SARS
SARS was a major pandemic in 2003. Hepatic dysfunction was described in patients with SARS. Up to 10% of patients had underlying chronic liver disease, particularly, chronic hepatitis B, probably owing to the geographic location of the SARS outbreak. Over 50% of patients developed abnormal liver function tests (mostly mild) and the majority recovered. However, in some studies, elevated liver function tests were associated with severe disease and, in particular, high ALT predicted ICU admission and death. This raised the possibility that SARS caused liver dysfunction rather than simply being associated with it.[35], [36], [37], [38], [39], [40], [41]
Liver abnormality in MERS
The first case of MERS-CoV infection was reported in 2012 in Saudi Arabia.42 Unlike SARS-CoV and SARS-CoV-2, MERS-CoV utilises dipeptidyl peptidase-4 (DPP-4), which is abundant in the liver, as the cell entry receptor.43 Low albumin was found to be an independent predictor of severe MERS-CoV infection.44 The liver biopsy in patients with MERS showed lobular lymphocytic infiltration and mild hydropic degeneration of hepatocytes.45 , 46 In patients with MERS, non-survivors had a higher incidence of liver injury than survivors (91.3% vs. 77.9%, respectively).47 , 48 Mortality was higher in patients with comorbidities.49 , 50
Clinical outcome of COVID-19
According to Wang and colleagues,22 disease progression manifested as increasing respiratory distress leading to pneumonia. In these patients, CT showed bilateral ground glass appearance and patchy pneumonia in almost 100% of patients. Most patients recovered with no sequalae. Overall, in patients with severe COVID-19, 19.6% developed ARDS, 16.7% had myocarditis which manifested as arrhythmias and 8.7% developed septic shock. However, this number was higher in patients admitted to the ICU; ARDS (61%), arrhythmias (44.4%), and shock (30.6%). These patients required mechanical ventilation and extracorporeal membrane oxygenation (ECMO).
Case fatality rates of 3.6–15% have been reported in 4,292 Chinese patients. Mortality was higher in men (3.25:1), those aged >75 years and those with comorbidities (diabetes mellitus, hypertension and cardiovascular disease). These comorbidities were noted in 48% of patients in a study by Zhou and colleagues51 reporting on 191 patients with COVID-19: 54 died (28.2% mortality) of whom 36 (66.6%) had underlying chronic disease. Fig. 1 illustrates the distribution of comorbidities in deceased patients. In the largest case series by Wu and colleagues,52 the overall mortality was 2.3%; however, the mortality rate was 49% in patients with critical disease. In a recent report from Italy by Remuzzi and colleagues,53 mortality related to COVID-19 was 6% (827 patients), with a male:female ratio of 4:1 and a mean age of 81 years among those who died. More than 60% of these patients had comorbidities. The median time from presentation to death was 14 days.4 , 22 Age-adjusted mortality in these 2 large series is shown in Fig. 2 .

Distribution of comorbidities in deceased patients with COVID-19.
COVID-19, coronavirus disease 2019.

Comparison of the case fatality rates of COVID-19 based on respective age groups in 2 large cohorts from China52 and Italy.53
COVID-19, coronavirus disease 2019. n.a., no data were available for age groups 50-59, 60-69, >90 years in the Chinese cohort.
According to a meta-analysis of 8 studies, including 46,248 patients, which analysed the prevalence of comorbidities in COVID-19, the most common comorbidities were hypertension (14–22%), followed by diabetes mellitus (6–11%), cardiovascular diseases (4–7%) and respiratory disease (1–3%).54 The mortality rate was higher in patients with hypertension (48%), followed by 21% in diabetics, 14% in patients with cardiovascular illness, 10% in those with chronic lung disease, and 4% each for malignancy, chronic kidney disease and cerebrovascular diseases.26 However, the mortality rate in patients with underlying chronic liver disease was 0–2%.55 In this analysis, hypertension (48% vs. 24%), diabetes (21% vs. 14%), and cardiovascular disease (14% vs. 4%) were more common in non-survivors. Fatty liver disease is likely seen as part of the metabolic syndrome in this group of patients, which can complicate the issue.
Another study from Wuhan reported on the characteristic features of deceased patients (n = 113). AST, ALT, alkaline phosphatase, GGT and bilirubin levels were significantly higher in non-survivors than survivors. Elevated AST (>40 U/L) was observed in 59 (52%) deceased and 25 (16%) recovered patients and likewise elevated ALT (>41 U/L) was found in 30 (27%) deceased and 30 (19%) recovered patients. Similarly, hypoalbuminemia (<32 g/L) was found in 74 (65%) deceased patients compared to 22 (14%) recovered patients. Serum bilirubin was 12.6 μmol and 8.4 μmol in the deceased and recovered patients, respectively. In a recent report by Chen et al., 13 (5%) patients with COVID-19 developed acute liver injury during the course of the illness of whom 10 (76.9%) died.26 Although the numbers are small, this conveys an important message on patients with COVID-19 and hepatic dysfunction.
Key point
Hepatic dysfunction was significantly more frequent in critically ill patients and was associated with poor outcome.
Diagnosis
Diagnosis of COVID-19 was based on Real time reverse transcription polymerase chain reaction (RT-PCR). In the case series described by Wang and colleagues22 centrifuged throat swab samples were used for testing. The total viral RNA was extracted within 2 hours using an RNA isolation kit. RT-PCR of the suspension was performed and amplification of Open reading frame (ORIF) and nucleocapsid protein were carried out using respective forward, reverse primers and the probe. Diagnosis were also obtained using nasal swabs, oral and rectal swabs. Interestingly, Xiao and colleagues20 showed patients with SARS CoV-2 related respiratory illness can continue to shed virus in stool even after a negative respiratory sample.
Management
Although the evidence is less clear, the current treatment recommendations include antiviral drugs, antibiotics, intravenous fluids and corticosteroids. Oseltamivir was utilised in 89.9% of patients in the Wuhan series. Remdisivir was though initially promising, a recent randomized control study did not show clinical benefit in COVID-19 except non significant faster clinical recovery. Moreover, liver injury was observed in 10-13 % of remdisivir treated group.56 Being an RNA virus, one would expect broad spectrum ribavirin to work; unfortunately, during the SARS outbreak, ribavirin was associated with significant toxicity including severe haemolysis. Interestingly, Omrani and colleagues57 found interferon alpha 2 A in combination with ribavirin to improve survival at day 14 (70% vs. 17%, p = 0.004) but not day 28 (30% vs. 17%, p = 0.054) during the MERS-CoV outbreak.
Lopinavir/ritonavir, approved for HIV infection showed in vitro activity against SARS-CoV and was beneficial in MERS-CoV.58 These drugs are being tried in COVID-19. Lopinavir, a protease inhibitor, has been shown to be effective in controlling SARS-CoV. Ritonavir was added to increase the trough level of lopinavir through CYP450 enzyme inhibition in liver. A recently published open labelled, randomised controlled trial on 199 patients with severe COVID-19 showed no benefit of lopinavir and ritonavir (99 patients). It was debated
Key point
Current treatment recommendations for COVID-19 include corticosteroids, antiviral drugs, antibiotics and intravenous fluids.
whether the trial should have been conducted in less sick patients and treatment should have been initiated in an earlier phase of COVID-19. In this study, 20.5% and 41% of patients had elevated AST and ALT prior to randomisation, respectively; however, the presence of cirrhosis, ALT or AST >5 times the upper limit normal were exclusion criteria in this trial. Increased bilirubin and elevated AST were noted in 3.2% and 2.1% of patients in the treatment group, respectively.59 Importantly, using ritonavir to inhibit CYP450 will increase the trough levels of calcineurin inhibitors, the most commonly used immunosuppression in solid organ transplant recipients, leading to potential drug toxicity.
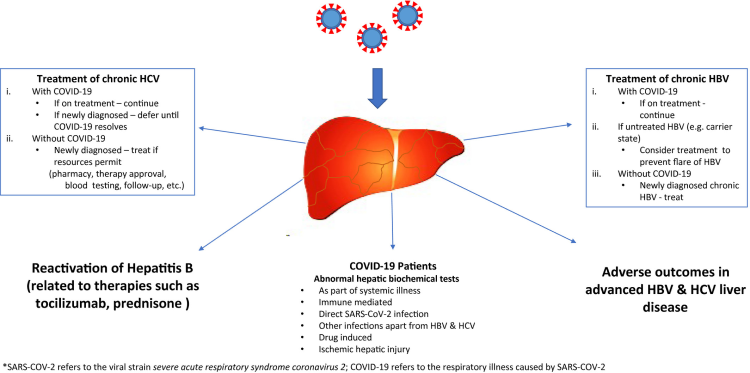
Antibiotics such as fluoroquinolones and third-generation cephalosporins were used to reduce secondary infection. Corticosteroids (methylprednisolone) have been used in patients with COVID-19 to curtail inflammation22 and, recently, dexamethasone has been found to reduce mortality. Their use can lead to the reactivation of chronic hepatitis B. Thus, HBsAg-positive patients should be given antiviral therapy and we recommend checking hepatitis B core antibody status and, if positive, treating patients with antivirals for the duration of steroid therapy.
Recently, Chen et al. constructed a 3-dimensional crystal structure model of SARS-CoV-2 proteases. Virtual screening of the active viral site demonstrated that hepatitis C NS5A inhibitors could be effective in controlling SARS-CoV-2. Ledipasvir and velpatasvir readily inhibited SARS-CoV proteases in their model. However, more evidence is required.
COVID-19 and HCC
Patients with underlying cancer are often immunosuppressed, as result of the natural history of the disease and chemotherapy. In a nationwide study of 1,590 cancer patients with COVID-19 across 575 hospitals in China, it was observed that patients with cancer were at higher risk of contracting SARS-CoV-2 infection and developing severe illness. They also had worse outcomes than those without cancer.61 Most patients with hepatocellular carcinoma (HCC) have underlying chronic liver disease and therefore, they fall under this high-risk category and are likely to have worse outcomes. AASLD currently recommends delaying HCC surveillance by 2 months; however, HCC-related treatments should be carried out without much delay.31 EASL recommends avoiding HCC surveillance in COVID-19-positive patients, postponing locoregional therapy and temporarily withholding immune checkpoint inhibitor therapy.62
COVID-19 and deceased donor transplantation
There has been a significant decline in cadaveric organ donation during the COVID-19 pandemic.63 This can affect patients awaiting liver or other solid organ transplantation, leading to increased waiting list mortality. There has been a recent debate on harvesting organs from SARS-CoV-2-positive donors, like the discussion around HCV-positive donors.64 However, the risk of disease transmission to the transplant team remains a major concern.65 This may be an interesting option in the future, when effective vaccination comes available.
Post-liver transplant COVID-19
COVID-19 leaves no stone unturned, including liver transplant recipients. A recent case report from Wuhan described a 37-year-old man with hepatitis B and HCC, who developed fever on the third day post transarterial chemoembolisation. He was initially treated with antibiotics and subsequently liver transplantation on day 7. His fever continued on day 9, and a CT scan of his chest showed hypostatic changes in both lung fields. A repeat CT on the third week showed bilateral ground glass appearance. His nasopharyngeal swab confirmed COVID-19. His tacrolimus dose was reduced and maintained under 10 ng/ml. His liver enzymes increased by the fourth week but settled gradually. His PCR remained positive for nearly 2 months and subsequently cleared.66
Another case of post-transplant COVID-19 was described recently. The patient underwent cadaveric liver transplantation in July 2017. He presented recently with high fever and developed severe COVID-19. His tacrolimus was discontinued for a month, but he received corticosteroid therapy. His allograft function remained normal.67
Some immunosuppressive drugs possess antiviral activity by virtue of their mechanism of action. Studies from SARS identified an interaction between SARS-CoV non-structural proteins and cyclophilins, resulting in modulation of T cell immune responses. In vitro studies showed that cyclosporine inhibited SARS-CoV at higher doses. However, its clinical utility was limited by its profound immunosuppressive effects.68 Similarly, mycophenolic acid exhibited potent antiviral properties against MERS-CoV in vitro.69 Interestingly, mTOR inhibitors (everolimus) showed effectiveness against SARS-CoV and MERS-CoV infections by blocking early viral entry and post-entry consequences.70 , 71 Although in vitro studies, the antiviral properties of these drugs may offer some protection against COVID-19 in transplant recipients, particularly to ameliorate disease severity.
Literature from SARS-CoV and MERS-CoV showed that post-liver transplant patients on immunosuppression were not at higher risk of mortality. Similar data on SARS-CoV-2 are very limited.72
Rapid clinical deterioration in COVID-19 is often due to a cytokine storm associated with elevated interleukin (IL)-6, IL-8 and tumour necrosis factor-alpha levels. The combined effects of SARS-CoV-2 infection and immunosuppression are not well established. However, stopping immunosuppressive medications in transplant patients may lead to rejection. In patients with COVID-19 on high dose steroids, the dose needs to be tapered and maintained at 10 mg/day. When there is lymphopenia, fever and worsening lung condition, azathioprine, mycophenolate and calcineurin inhibitor doses need to be reduced but not stopped. Caution needs to be exercised when considering initiation of steroids or other immunosuppressive therapy in patients with severe alcoholic hepatitis, autoimmune hepatitis etc.31 Patients on immunosuppression may be more infectious as they have higher viral titres.73
The American Society of Transplantation has provided a few recommendations specifically for those awaiting liver transplantation and transplant recipients during the current pandemic. The recommendations include patient education, hand hygiene and social distancing, provision for patients to contact the transplant centre via telephone if they develop fever, cough or flu-like symptoms. Each hospital should provide layout protocols for managing these high-risk patients. Allograft function and drug interactions should be carefully monitored in transplant recipients with COVID-19, because ritonavir can potentially inhibit the CYP34A enzyme, leading to increasing trough levels of mTOR and calcineurin inhibitors, and possibly drug toxicity. In addition, they have recommended postponing elective surgeries including living donor transplantation and non-urgent deceased donor transplantations in areas with a high prevalence of COVID-19. In addition, potential deceased donors should be adequately tested for SARS-CoV-2 with nucleic acid assays.73
Conclusion
COVID-19 is currently a pandemic, with an overall mortality rate of 2–6% in infected patients, which increases with age and comorbidities. COVID-19 causes pneumonia, but hepatic dysfunction can occur in severe cases and is associated with fatal outcome. Cases of severe acute liver injury have been reported with higher mortality. Larger studies with long-term follow-up are required to characterise the extent and cause of liver damage in COVID-19. The effects of COVID-19 on underlying chronic liver disease require detailed evaluation, with further research warranted in this area.
Abbreviations
ACE2, angiotensin-converting enzyme 2; ALT, alanine aminotransferase; ARDS, acute respiratory distress syndrome; AST, aspartate aminotransferase; COVID-19, coronavirus disease 2019; GGT, gamma glutamyltransferase; HCC, hepatocellular carcinoma; ICU, intensive care unit; MERS, Middle East respiratory syndrome; MERS-CoV, MERS coronavirus; NAFLD, non-alcoholic fatty liver disease; RT-PCR, reverse transcription PCR; SARS, severe acute respiratory syndrome; SARS-CoV, SARS coronavirus; SARS-CoV-2, SARS coronavirus 2; SIRS, systemic inflammatory response syndrome; WHO, World Health Organization.
Financial support
The authors received no financial support to produce this manuscript.
Authors’ contributions
Dinesh Jothimani: Conceptualization; Project Administration; Supervision; Writing -original draft; Writing – review & editing. Radhika Venugopal: Data curation; Resources; Software; Writing – review & editing. Mohammed Forhad Abedin: Resources; Writing – review & editing; Ilankumaran Kaliamoorthy: Supervision; Validation; Writing – review & editing; Mohamed Rela: Conceptualization; Supervision; Writing – review & editing.
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2020.06.006.
Supplementary data
disclosures.pdf:Click here to view.(185K, pdf)Go to:
References
1. Benvenuto D., Giovanetti M., Ciccozzi A., Spoto S., Angeletti S., Ciccozzi M. The 2019-new coronavirus epidemic: evidence for virus evolution. J Med Virol. 2020;92(4):455–459. [PMC free article] [PubMed] [Google Scholar]
2. Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. [PMC free article] [PubMed] [Google Scholar]
3. Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. China novel coronavirus investigating and research team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. [PMC free article] [PubMed] [Google Scholar]
4. Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. [PMC free article] [PubMed] [Google Scholar]
5. Chen J. Pathogenicity and transmissibility of 2019-nCoV-A quick overview and comparison with other emerging viruses. Microbes Infect. 2020;22(2):69–71. [PMC free article] [PubMed] [Google Scholar]
6. World Health Organization Situation report – 120; Coronavirus disease 2019 (COVID-19) 2020. www.who.int Available at. Accessed May 20, 2020.
7. Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. [PMC free article] [PubMed] [Google Scholar]
8. Guo Y.R., Cao Q.D., Hong Z.S., Tan Y.Y., Chen S.D., Jin H.J. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak – an update on the status. Mil Med Res. 2020;7(1):11. [PMC free article] [PubMed] [Google Scholar]
9. Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. [PMC free article] [PubMed] [Google Scholar]
10. Chai X., Hu L., Zhang Y., Han W., Lu Z., Ke A. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. BioRxiv. 2020 doi: 10.1101/2020.02.03.931766. [CrossRef] [Google Scholar]
11. Zhao S., Lin Q., Ran J., Musa S.S., Yang G., Wang W. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: a data-driven analysis in the early phase of the outbreak. Int J Infect Dis. 2020;92:214–217. [PMC free article] [PubMed] [Google Scholar]
12. Zhou C. Evaluating new evidence in the early dynamics of the novel coronavirus COVID-19 outbreak in Wuhan, China with real time domestic traffic and potential asymptomatic transmissions. medRxiv. 2020 doi: 10.1101/2020.02.15.20023440. [CrossRef] [Google Scholar]
13. van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564–1567. [PMC free article] [PubMed] [Google Scholar]
14. Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. [PMC free article] [PubMed] [Google Scholar]
15. Leung W.K., To K.F., Chan P.K., Chan H.L., Wu A.K., Lee N. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology. 2003;125(4):1011–1017. [PMC free article] [PubMed] [Google Scholar]
16. Peiris J.S., Chu C.M., Cheng V.C., Chan K.S., Hung I.F., Poon L.L., HKU/UCH SARS Study Group Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361(9371):1767–1772. [PMC free article] [PubMed] [Google Scholar]
17. Lee N., Hui D., Wu A., Chan P., Cameron P., Joynt G.M. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348(20):1986–1994. [PubMed] [Google Scholar]
18. Tian Y., Rong L., Nian W., He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther. 2020;51(9):843–851. [PMC free article] [PubMed] [Google Scholar]
19. Zhang W., Du R.H., Li B., Zheng X.S., Yang X.L., Hu B. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9(1):386–389. [PMC free article] [PubMed] [Google Scholar]
20. Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158(6):1831–1833.e3. [PMC free article] [PubMed] [Google Scholar]
21. Yeo C., Kaushal S., Yeo D. Enteric involvement of coronaviruses: is faecal-oral transmission of SARS-CoV-2 possible. Lancet Gastroenterol Hepatol. 2020;5(4):335–337. [PMC free article] [PubMed] [Google Scholar]
22. Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. [PMC free article] [PubMed] [Google Scholar]
23. Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., China Medical Treatment Expert Group for Covid-19 Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. [PMC free article] [PubMed] [Google Scholar]
24. Zhang C., Shi L., Wang F.S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5(5):428–430. [PMC free article] [PubMed] [Google Scholar]
25. Xu X.W., Wu X.X., Jiang X.G., Xu K.J., Ying L.J., Ma C.L. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. [PMC free article] [PubMed] [Google Scholar]
26. Chen T., Wu D., Chen H., Yan W., Yang D., Chen G. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. [PMC free article] [PubMed] [Google Scholar]
27. Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. [PMC free article] [PubMed] [Google Scholar]
28. Fan Z., Chen L., Li J., Tian C., Zhang Y., Huang S. Clinical features of COVID-19 related liver damage. medRxiv. 2020 doi: 10.1101/2020.02.26.20026971. [CrossRef] [Google Scholar]
29. Alqahtani S.A., Schattenberg J.M. Liver injury in COVID-19: The current evidence. United European Gastroenterol J. 2020;8(5):509–519. [PMC free article] [PubMed] [Google Scholar]
30. Xu L., Liu J., Lu M., Yang D., Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40(5):998–1004. [PMC free article] [PubMed] [Google Scholar]
31. Clinical insights for hepatology and liver transplant providers during the covid-19 pandemic American Association for the Study of Liver Diseases. www.aasld.org Available at. Released March 23, 2020. [PMC free article] [PubMed]
32. Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. [PMC free article] [PubMed] [Google Scholar]
33. Tian S., Xiong Y., Liu H., Niu H., Guo J., Liao M. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33(6):1007–1014. [PMC free article] [PubMed] [Google Scholar]
34. Chau T.N., Lee K.C., Yao H., Tsang T.Y., Chow T.C., Yeung Y.C. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004;39(2):302–310. [PMC free article] [PubMed] [Google Scholar]
35. Chang H.L., Chen K.T., Lai S.K., Kuo H.W., Su I.J., Lin R.S. Hematological and biochemical factors predicting SARS fatality in Taiwan. J Formos Med Assoc. 2006;105(6):439–450. [PMC free article] [PubMed] [Google Scholar]
36. Chan H.L., Kwan A.C., To K.F., Lai S.T., Chan P.K., Leung W.K. Clinical significance of hepatic derangement in severe acute respiratory syndrome. World J Gastroenterol. 2005;11(14):2148–2153. [PMC free article] [PubMed] [Google Scholar]
37. Yang Z., Xu M., Yi J.Q., Jia W.D. Clinical characteristics and mechanism of liver damage in patients with severe acute respiratory syndrome. Hepatobiliary Pancreat Dis Int. 2005;4(1):60–63. [PubMed] [Google Scholar]
38. Wu K.L., Lu S.N., Changchien C.S., Chiu K.W., Kuo C.H., Chuah S.K. Sequential changes of serum aminotransferase levels in patients with severe acute respiratory syndrome. Am J Trop Med Hyg. 2004;71(2):125–128. [PubMed] [Google Scholar]
39. Duan Z.P., Chen Y., Zhang J., Zhao J., Lang Z.W., Meng F.K. Clinical characteristics and mechanism of liver injury in patients with severe acute respiratory syndromeZhonghua Gan Zang Bing Za Zhi. 2003;11(8):493–496. Chinese. [PubMed] [Google Scholar]
40. Chan H.L., Leung W.K., To K.F., Chan P.K., Lee N., Wu A. Retrospective analysis of liver function derangement in severe acute respiratory syndrome. Am J Med. 2004;116(8):566–567. [PMC free article] [PubMed] [Google Scholar]
41. Peiris J.S., Lai S.T., Poon L.L., Guan Y., Yam L.Y., Lim W., SARS study group Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361(9366):1319–1325. [PMC free article] [PubMed] [Google Scholar]
42. Raj V.S., Mou H., Smits S.L., Dekkers D.H., Müller M.A., Dijkman R. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495(7440):251–254. [PMC free article] [PubMed] [Google Scholar]
43. Boonacker E., Van Noorden C.J. The multifunctional or moonlighting protein CD26/DPPIV. Eur J Cell Biol. 2003;82(2):53–73. [PubMed] [Google Scholar]
44. Saad M., Omrani A.S., Baig K., Bahloul A., Elzein F., Matin M.A. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis. 2014;29:301–306. [PMC free article] [PubMed] [Google Scholar]
45. Ng D.L., Al Hosani F., Keating M.K., Gerber S.I., Jones T.L., Metcalfe M.G. Clinicopathologic, immunohistochemical, and ultrastructural findings of a fatal case of Middle East respiratory syndrome coronavirus infection in the United Arab Emirates, April 2014. Am J Pathol. 2016;186(3):652–658. [PMC free article] [PubMed] [Google Scholar]
46. Alsaad K.O., Hajeer A.H., Al Balwi M., Al Moaiqel M., Al Oudah N., Al Ajlan A. Histopathology of Middle East respiratory syndrome coronovirus (MERS-CoV) infection – clinicopathological and ultrastructural study. Histopathology. 2018;72(3):516–524. [PMC free article] [PubMed] [Google Scholar]
47. Arabi Y.M., Al-Omari A., Mandourah Y., Al-Hameed F., Sindi A.A., Alraddadi B., Saudi Critical Care Trial Group Critically ill patients with the Middle East respiratory syndrome: a multicenter retrospective cohort study. Crit Care Med. 2017;45(10):1683–1695. [PubMed] [Google Scholar]
48. Hwang S.M., Na B.J., Jung Y., Lim H.S., Seo J.E., Park S.A. Clinical and laboratory findings of Middle East respiratory syndrome coronavirus infection. Jpn J Infect Dis. 2019;72(3):160–167. [PubMed] [Google Scholar]
49. Assiri A., Al-Tawfiq J.A., Al-Rabeeah A.A., Al-Rabiah F.A., Al-Hajjar S., Al-Barrak A. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13(9):752–761. [PMC free article] [PubMed] [Google Scholar]
50. Arabi Y.M., Arifi A.A., Balkhy H.H., Najm H., Aldawood A.S., Ghabashi A. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160(6):389–397. [PubMed] [Google Scholar]
51. Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. [PMC free article] [PubMed] [Google Scholar]
52. Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. [PubMed] [Google Scholar]
53. Remuzzi A., Remuzzi G. COVID-19 and Italy: what next. Lancet. 2020;395(10231):1225–1228. [PMC free article] [PubMed] [Google Scholar]
54. Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. [PMC free article] [PubMed] [Google Scholar]
55. Bangash M.N., Patel J., Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;5(6):529–530. [PMC free article] [PubMed] [Google Scholar]
56. Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. [PMC free article] [PubMed] [Google Scholar]
57. Omrani A.S., Saad M.M., Baig K., Bahloul A., Abdul-Matin M., Alaidaroos A.Y. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis. 2014;14(11):1090–1095. [PMC free article] [PubMed] [Google Scholar]
58. Chu C.M., Cheng V.C., Hung I.F., Wong M.M., Chan K.H., Chan K.S., HKU/UCH SARS Study Group Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59(3):252–256. [PMC free article] [PubMed] [Google Scholar]
59. Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. [PMC free article] [PubMed] [Google Scholar]
60. Chen Y.W., Yiu C.B., Wong K.Y. Prediction of the SARS-CoV-2 (2019-nCoV) 3C-like protease (3CL pro) structure: virtual screening reveals velpatasvir, ledipasvir, and other drug repurposing candidates. F1000Res. 2020;9:129. [PMC free article] [PubMed] [Google Scholar]
61. Liang W., Guan W., Chen R., Wang W., Li J., Xu K. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. [PMC free article] [PubMed] [Google Scholar]
62. Boettler T., Newsome P.N., Mondelli M.U., Maticic M., Cordero E., Cornberg M. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep. 2020;2(3):100113. [PMC free article] [PubMed] [Google Scholar]
63. Loupy A., Aubert O., Reese P.P., Bastien O., Bayer F., Jacquelinet C. Organ procurement and transplantation during the COVID-19 pandemic. Lancet. 2020;395(10237):e95–e96. [PMC free article] [PubMed] [Google Scholar]
64. Kates O.S., Fisher C.E., Rakita R.M., Reyes J.D., Limaye A.P. Use of SARS-CoV-2 infected deceased organ donors: should we always “just say no?” Am J Transplant. 2020;20(7):1787–1794. [PMC free article] [PubMed] [Google Scholar]
65. Shah M.B., Lynch R.J., El-Haddad H., Doby B., Brockmeier D., Goldberg D.S. Utilization of deceased donors during a pandemic: an argument against using SARS-CoV-2 positive donors. Am J Transplant. 2020;20(7):1795–1799. [PMC free article] [PubMed] [Google Scholar]
66. Qin J., Wang H., Qin X., Zhang P., Zhu L., Cai J. Perioperative presentation of COVID-19 disease in a liver transplant recipient. Hepatology. 2020 doi: 10.1002/hep.31257. Epub ahead of print. [PubMed] [CrossRef] [Google Scholar]
67. Liu B., Wang Y., Zhao Y., Shi H., Zeng F., Chen Z. Succssful treatment of severe COVID-19 pneumonia in a liver transplant recipient. Am J Transplant. 2020;20(7):1891–1895. [PubMed] [Google Scholar]
68. Tanaka Y., Sato Y., Sasaki T. Suppression of coronavirus replication by cyclophilin inhibitors. Viruses. 2013;5(5):1250–1260. [PMC free article] [PubMed] [Google Scholar]
69. Chan J.F., Chan K.H., Kao R.Y., To K.K., Zheng B.J., Li C.P. Broad-spectrum antivirals for the emerging Middle East respiratory syndrome coronavirus. J Infect. 2013;67(6):606–616. [PMC free article] [PubMed] [Google Scholar]
70. Kindrachuk J., Ork B., Hart B.J., Mazur S., Holbrook M.R., Frieman M.B. Antiviral potential of ERK/MAPK and PI3K/AKT/mTOR signaling modulation for Middle East respiratory syndrome coronavirus infection as identified by temporal kinome analysis. Antimicrobial Agents Chemother. 2015;59(2):1088–1099. [PMC free article] [PubMed] [Google Scholar]
71. Zumla A., Chan J.F., Azhar E.I., Hui D.S., Yuen K.Y. Coronaviruses – drug discovery and therapeutic options. Nat Rev Drug Discov. 2016;15(5):327–347. [PMC free article] [PubMed] [Google Scholar]
72. D’Antiga L. Coronaviruses and immunosuppressed patients. The facts during the third epidemic. Liver Transpl. 2020;26(6):832–834. [PubMed] [Google Scholar]
73. American Society of Transplantation 2019-nCoV (Coronavirus): FAQs for organ donation and transplantation. www.myast.org Updated 20 Mar 2020. Available at. Accessed May 20, 2020.
74. Li L., Li S., Xu M., Li L., Li S., Xu M. Risk factors related to hepatic injury in patients with corona virus disease 2019. medRxiv. 2020 doi: 10.1101/2020.02.28.20028514. [CrossRef] [Google Scholar]
75. Cai Q., Huang D., Ou P., Yu H., Zhu Z., Xia Z. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. medRxiv. 2020 doi: 10.1101/2020.02.17.20024018. [PubMed] [CrossRef] [Google Scholar]
76. Cao W. Clinical features and laboratory inspection of novel coronavirus pneumonia (COVID-19) in Xiangyang, Hubei. medRxiv. 2020 doi: 10.1101/2020.02.23.20026963. [CrossRef] [Google Scholar]
77. Shi H., Han X., Jiang N., Cao Y., Alwalid O., Gu J. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425–434. [PMC free article] [PubMed] [Google Scholar
]78. Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):1–11. [PMC free article] [PubMed] [Google Scholar]
79. Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., COVID-19 Lombardy ICU Network Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323(16):1574–1581. [PMC free article] [PubMed] [Google Scholar]
80. Arentz M., Yim E., Klaff L., Lokhandwala S., Riedo F.X., Chong M. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA. 2020;323(16):1612–1614. [PMC free article] [PubMed] [Google Scholar]
- Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19).[J Pathol. 2020]
- Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19.[J Med Virol. 2020]
- Renin-angiotensin-aldosterone system and COVID-19 infection.[Ann Endocrinol (Paris). 2020]
- COVID-19: a conundrum to decipher.[Eur Rev Med Pharmacol Sci. 2020]
- Natural Flavonoids as Potential Angiotensin-Converting Enzyme 2 Inhibitors for Anti-SARS-CoV-2.[Molecules. 2020]
Cited by other articles in PMC
- Harnessing reactive oxygen/nitrogen species and inflammation: Nanodrugs for liver injury[Materials Today Bio. 2022]
- Liver Injury in Patients with COVID-19 without Underlying Liver Disease[Journal of Clinical Medicine. …]
- A Case of Hepatotoxicity After Receiving a COVID-19 Vaccine[Cureus. 2021]
- A case report of COVID-19 evoked cholangitic liver abscess[Egyptian Liver Journal. 2022]
- Mechanism of SARS-CoV-2 Invasion into the Liver and Hepatic Injury in Patients with COVID-19[Mediterranean Journal of Hemat…]
- COVID-19 and the liverCOVID-19 and the liverElsevier Public Health Emergency Collection. 2020 Nov; 73(5)1231
- Case report: Acute pericarditis as a primary presentation of COVID-19Case report: Acute pericarditis as a primary presentation of COVID-19BMJ Case Reports. 2020; 13(8)
- Acute Kidney Injury in COVID-19Acute Kidney Injury in COVID-19International Journal of Molecular Sciences. 2021 Aug; 22(15)
- COVID-19 vaccination followed by activation of glomerular diseases: does associa…COVID-19 vaccination followed by activation of glomerular diseases: does association equal causation?Elsevier Public Health Emergency Collection. 2021 Nov; 100(5)959
- Electrocardiographic Changes in COVID-19 Patients: A Hospital-based Descriptive …Electrocardiographic Changes in COVID-19 Patients: A Hospital-based Descriptive StudyIndian Journal of Critical Care Medicine : Peer-reviewed, Official Publication of Indian Society of Critical Care Medicine. 2022 Jan; 26(1)43