Authors: Yan-Mei ChenYuanting ZhengYing YuYunzhi WangQingxia HuangFeng QianLei SunZhi-Gang SongZiyin ChenJinwen FengYanpeng AnJingcheng YangZhenqiang SuShanyue SunFahui DaiQinsheng ChenQinwei LuPengcheng LiYun LingZhong YangHuiru TangLeming ShiLi JinEdward C HolmesChen DingTong-Yu ZhuYong-Zhen Zhang
Abstract
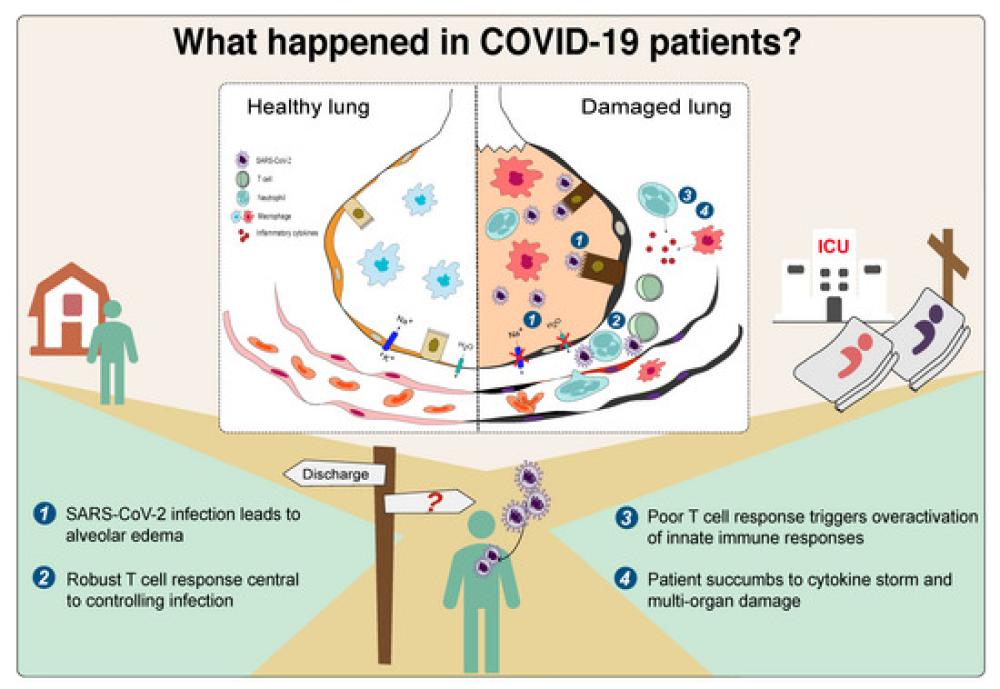
COVID-19 is characterized by dysregulated immune responses, metabolic dysfunction and adverse effects on the function of multiple organs. To understand host responses to COVID-19 pathophysiology, we combined transcriptomics, proteomics, and metabolomics to identify molecular markers in peripheral blood and plasma samples of 66 COVID-19-infected patients experiencing a range of disease severities and 17 healthy controls. A large number of expressed genes, proteins, metabolites, and extracellular RNAs (exRNAs) exhibit strong associations with various clinical parameters. Multiple sets of tissue-specific proteins and exRNAs varied significantly in both mild and severe patients suggesting a potential impact on tissue function. Chronic activation of neutrophils, IFN-I signaling, and a high level of inflammatory cytokines were observed in patients with severe disease progression. In contrast, COVID-19-infected patients experiencing milder disease symptoms showed robust T-cell responses. Finally, we identified genes, proteins, and exRNAs as potential biomarkers that might assist in predicting the prognosis of SARS-CoV-2 infection. These data refine our understanding of the pathophysiology and clinical progress of COVID-19.
Proteomics, metabolomics and RNAseq data map immune responses in COVID-19 patients with different disease severity, revealing molecular makers associated with disease progression and alterations of tissue-specific proteins.
- A multi-omics profiling of the host response to SARS-CoV2 infection in 66 clinically diagnosed and laboratory confirmed COVID-19 patients and 17 uninfected controls.
- Significant correlations between multi-omics data and key clinical parameters.
- Alteration of tissue-specific proteins and exRNAs.
- Enhanced activation of immune responses is associated with COVID-19 pathogenesis.
- Biomarkers to predict COVID-19 clinical outcomes pending clinical validation as prospective marker.
Introduction
Coronaviruses (family Coronaviridae) are a diverse group of positive-sense single-stranded RNA viruses with enveloped virions (Masters & Perlman, 2013; Cui et al, 2019). Coronaviruses are well known due to the emergence of Severe Acute Respiratory Syndrome (SARS) in 2002–2003 and Middle East Respiratory Syndrome (MERS) in 2012, both of which caused thousands of cases in multiple countries (Ksiazek et al, 2003; Bermingham et al, 2012; Cui et al, 2019). Coronaviruses naturally infect a broad range of vertebrate hosts including mammals and birds (Cui et al, 2019). As coronavirus primarily target epithelial cells, they are generally associated with gastrointestinal and respiratory infections (Masters & Perlman, 2013; Cui et al, 2019). In addition, they cause hepatic and neurological diseases of varying severity (Masters & Perlman, 2013).
The world is currently experiencing a disease pandemic (COVID-19) caused by a newly identified coronavirus called SARS-CoV-2 (Wu et al, 2020a). At the time of writing, there have been more than ~25 million cases of SARS-CoV-2 and ~830,000 deaths globally (WHO, 2020). The disease leads to both mild and severe respiratory manifestations, with the latter prominent in the elderly and those with underlying medical conditions such as cardiovascular and chronic respiratory disease, diabetes, and cancer (Guan et al., 2020). In addition to respiratory syndrome, mild gastrointestinal and/or cardiovascular symptoms and neurological manifestations have been documented in hospitalized COVID-19-infected patients (Gupta et al, 2020; Mao et al, 2020). These data point to the complexity of COVID-19 pathogenesis, especially in patients experiencing severe disease.
SARS-CoV-2 is able to use angiotensin-converting enzyme 2 (ACE 2) as a receptor for cell entry (Hoffmann et al, 2020; Zheng et al, 2020a; Zhou et al, 2020b). Aside from lungs, ACE2 is expressed in other organs including heart, liver, kidney, pancreas, and small intestines (Li et al, 2020; Liu et al, 2020; Zou et al, 2020; Chen et al, 2020a). More recently, ACE2 expression has also been found in Leydig cells in the testes (Li et al, 2020; Wang & Xu, 2020) and neurological tissue (Baig et al, 2020; Bullen et al, 2020; Xu & Lazartigues, 2020). As such, it is possible that these organs might also be infected by SARS-CoV-2, and recent autopsy studies have also revealed multi-organ damage including heart, liver, intestine, pancreas, brain, kidney, and spleen in fatal COVID-19-infected patients (Lax et al, 2020; Menter et al, 2020; Varga et al, 2020; Wichmann et al, 2020; Wang et al, 2020c). The host immune response to SARS-CoV-2 may also impact pathogenicity, resulting in severe tissue damage and, occasionally, death (Tay et al, 2020). Indeed, several studies have reported lymphopenia, exhausted lymphocytes, and cytokine storms in COVID-19-infected patients, especially those with severe symptoms (Blanco-Melo et al, 2020; Cao, 2020; Chua et al, 2020; Liao et al, 2020). Numerous clinical studies have also observed the elevation of lactate dehydrogenase (LDH), IL-6, troponin I, inflammatory markers, and D-dimer in COVID-19-infected patients (Zhou et al, 2020a; Wang et al, 2020b). However, despite the enormous burden of morbidity and mortality due to COVID-19, we know little about its pathophysiology, even though this establishes the basis for successful clinical practice, vaccine development, and drug discovery.
Using a multi-omics approach employing cutting-edge transcriptomic, proteomic, and metabolomic technologies, we identified significant molecular alterations in patients with COVID-19 compared with uninfected controls in this study. Our results refine the molecular view of COVID-19 pathophysiology associated with disease progression and clinical outcome.
For More Information: https://www.embopress.org/doi/full/10.15252/embj.2020105896