Both of the mRNA vaccines available in the US are highly effective against severe COVID-19, but recent studies suggest that Moderna’s elicits a stronger immune response and might be better at preventing breakthrough infections.
Authors: Alejandra Manjarrez Sep 24, 2021
TeamPfizer and #TeamModerna are two of the captions often found next to selfies of proud newly vaccinated people on social media. And both groups do have a reason to celebrate as the two mRNA vaccines available in the US have proved to be highly effective against severe COVID-19.https://platform.twitter.com/embed/Tweet.html?dnt=false&embedId=twitter-widget-
Research published in recent weeks, however, suggests the Moderna vaccine’s advantage in terms of long-term protection. In multiple independent studies, significantly lower antibody levels and more vaccine breakthrough infections have been detected in the Pfizer/BioNTech-vaccinated population. These could be explained by a myriad of slight differences between the vaccines themselves: their distinct dosages, the intervals between the two doses, or the composition of the lipid nanoparticles used in each one. For now, all possibilities remain open.
By comparing vaccines and exploring the basis of their differences, “we are going to learn a lot in terms of what are the optimal strategies for vaccination,” says immunologist Alessandro Sette of the La Jolla Institute for Immunology in California, adding that there may not be one clear winner. “Some platforms may have some advantage[s] in terms of how fast the response develops, other[s] may have advantage[s] in how durable or how strong the response is,” he says.
Breakthrough infections: advantage Moderna
On the surface, the vaccines are very similar. Both are composed of lipid nanoparticles filled with mRNA. When these mRNAs get into cells, the cells begin to produce the non-infectious viral spike proteins they code for, which then trigger an immune response that trains the immune system to recognize the virus’s spiky exterior, so that it can launch a swift defense against the real virus.
And at first, these similarities seemed to extend to how well each vaccines worked. Early results found that both Pfizer/BioNTech’s and Moderna’s mRNA-based vaccines were outstanding at preventing COVID-19—results from Phase 3 clinical trials, which followed up participants for two months after they received their second shot, demonstrated an efficacy of 95 percent for Pfizer/BioNTech and 94.5 percent for Moderna. In the US, near 100 million people have been fully vaccinated with Pfizer/BioNTech and near 68 million with Moderna.
See “The Promise of mRNA Vaccines”
But more recent data have started to suggest a divergence in performance. For instance, a medRxiv preprint first published August 8 reports significantly fewer breakthrough infections among Moderna vaccine recipients than a similar cohort of participants that received Pfizer/BioNTech’s.
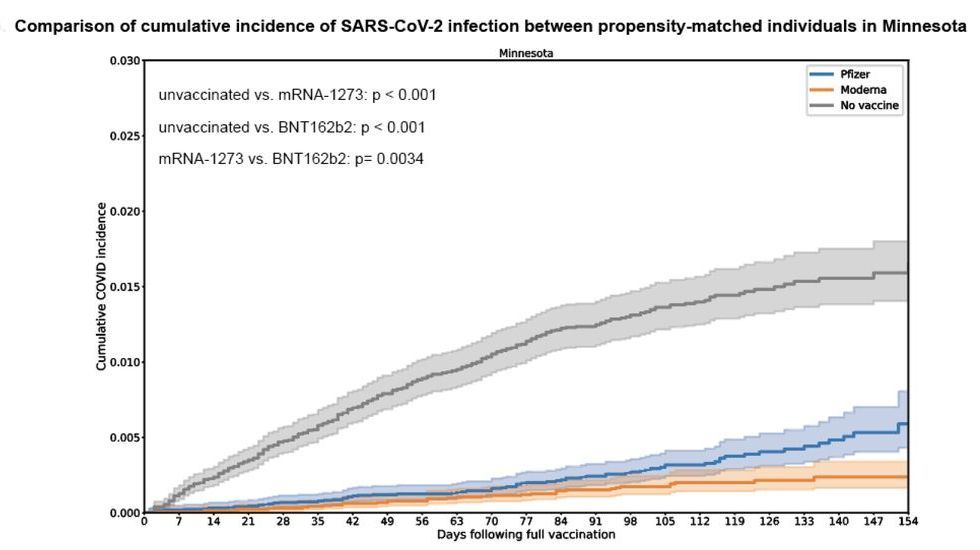
The study analyzed data from people who underwent SARS-CoV-2 PCR testing at the Mayo Clinic Health System in Minnesota from January to July 2021. Within the population available, they matched patients of similar age, same sex, race, ethnicity, SARS-CoV-2 testing history, and date of vaccination (if applicable) from three different groups: those vaccinated with Moderna, those with Pfizer/BioNTech, and those unvaccinated. This matching exercise resulted in groups of between 21,000 and 25,000 individuals for each of the three vaccination categories.
The team then assessed how the mRNA vaccines protected individuals from COVID-19 and found that, while SARS-CoV-2 positive test results were rare among those vaccinated compared to the unvaccinated group (38 infections in those who received Moderna’s, 72 for Pfizer/BioNTech’s, and 321 among the unvaccinated), the Moderna cohort had significantly fewer of them. The estimated effectiveness of Moderna at preventing infections was 86 percent versus 76 percent in Pfizer/BioNTech, and when they expanded the data to four other US states, they saw a similar trend: the numbers altogether showed a two-fold risk reduction of breakthrough infections conferred by Moderna’s vaccine when compared to Pfizer/BioNTech’s.
The numbers altogether showed a two-fold risk reduction of breakthrough infections conferred by Moderna’s vaccine when compared to Pfizer/BioNTech’s.
Interestingly, vaccine effectiveness against infection decreased notably in July for both vaccines, but more pronouncedly for Pfizer/BioNTech’s. It is not clear whether this was due to the prevalence of the Delta variant or to the waning of the antibodies after vaccination, as while cohorts were matched with regards to vaccination timing, analyses to examine effectiveness over time were not performed. AJ Venkatakrishnan, vice president of scientific research at the artificial intelligence company nference and coauthor of the paper, says that these findings are aimed at “provoking future research to look at durability of the vaccines. . . and to also understand, at a clinical level, how protective these vaccines are against specific variants.”
