Stefania Campana; Claudia De Pasquale; Giacomo Sidoti Migliore;, et. al. J Immunol (2022) 209 (4): 655–659. https://doi.org/10.4049/jimmunol.2200021
Abstract
Proinflammatory monocytes play a preponderant role in the development of a cytokine storm causing fatal consequences in coronavirus disease 2019 (COVID-19) patients, highlighting the importance of analyzing in more detail monocyte distribution in these patients. In this study, we identified an atypical monocyte subpopulation expressing CD56 molecules that showed a low level of HLA-DR and high level of l-selectin. They released higher amounts of TNF-α and IL-6 and expressed genes associated with an excessive inflammatory process. Remarkably, the frequency of CD56+ monocytes inversely correlated with that of CD16+ monocytes and a high CD56+/CD16+monocyte ratio was associated with both disease severity and mortality, as well as with serum concentration of type I IFN, a factor able to induce the appearance of CD56+ monocytes. In conclusion, severe COVID-19 is characterized by the abundance of hyperinflammatory CD56+ monocytes, which could represent a novel marker with prognostic significance and, possibly, a therapeutic target for controlling the inflammatory process occurring during COVID-19.
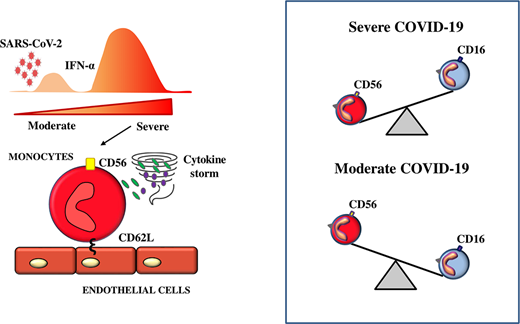
Visual Abstract
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has emerged as a novel enveloped RNA virus that rapidly spread worldwide causing the coronavirus disease 2019 (COVID-19) pandemic. The immunological mechanisms that govern COVID-19 pathophysiology are not yet completely understood, and the factors triggering severe illness appear largely associated with an overwhelming systemic inflammatory response characterized by high serum levels of proinflammatory cytokines (1).
Monocytes are heterogeneous innate myeloid cells that play different key roles in the regulation of the inflammatory process: classical monocytes (CD14+CD16−) are critical for the initial inflammatory response, and non-classical monocytes (CD14dimCD16+) maintain vascular homeostasis and display anti-inflammatory activity (2), whereas the intermediate subset (CD14+CD16+) represents a transitional population between the classical and nonclassical monocyte subsets (3). Severe COVID-19 is marked by both quantitative and qualitative changes within the monocyte population (4, 5). Activated monocytes migrating from peripheral blood (PB) have been shown to infiltrate the lungs in patients with SARS-CoV-2 infection (6) and are prominent in bronchoalveolar lavage fluids from patients with severe COVID-19 (7). Altogether, these results highlight the importance of analyzing in more detail monocyte distribution in COVID-19 patients to identify the specific subsets contributing to the inflammatory storm associated with severe disease.
In the current study, we identified a circulating atypical monocyte subpopulation characterized by the expression of the transmembrane molecule CD56 (neural cell adhesion molecule) that displayed a consistent hyperinflammatory profile. Interestingly, CD56+ monocytes inversely correlated with the frequency of anti-inflammatory CD16+ monocytes and were significantly associated with disease severity.
Materials and Methods
Sample collection
A total of 59 SARS-CoV-2–infected patients were enrolled in the study and their baseline characteristics are described in Supplemental Table I. PBMCs and sera samples were collected within 72 h from hospitalization at the University Hospital G. Martino (University of Messina). Ethical approval was obtained from the Ethics Committee of the University Hospital G. Martino, Messina (no. 7920, December12, 2020).
Cell sorting and culture assay
Monocytes were isolated from PBMCs of healthy donors (HDs) and patients affected by COVID-19 either requiring or not admission to the intensive care unit (ICU).
PB Plasmacytoid Dendritic cells (pDCs) were analyzed for the expression of PD-L1 molecules in both HDs and COVID-19 patients.
Expression of CD56 and CD16 was assessed on HD monocytes cultured for 3 d in the presence of recombinant human IFN-α (1000 U/ml, Sigma-Aldrich) or medium containing 10% patient serum with or without neutralizing Abs anti-human IFN-α, anti-human IFN-αR2, or anti-human IL-6 (ab9386, Abcam; MAB4015-100, R&D systems; and MAB2061R, R&D Systems, respectively).
Expression of CD56 and CD16 was also assessed on both HD and ICU patient monocytes following 24 h of culture in the presence of corticosteroid (hydrocortisone at 5 ng/ml, Lonza). TNF-α and IL-6 production was assessed on monocytes stimulated with either LPS (1 µg/ml, Sigma-Aldrich), corticosteroid (5 ng/ml), or R848 (10 µg/ml, Sigma-Aldrich) for 4 h. The mAbs used in this study are summarized in Supplemental Table II.
ImageStream analysis
Monocytes and NK cells from both HDs and ICU patients were stained with CD14, CD56, and nuclear dye DRAQ5 and then acquired by Amnis ImageStream flow cytometer.
Quantitative real-time PCR
Total RNA was isolated from CD56+ monocytes of ICU patients and from HD monocytes as control (11828665001, Roche), and gene expression of ATF3-activating transcription factor 3 (Hs00231069_m1), NFIL3-NF, IL-3 regulated (Hs00705412_s1), and HIVEP2-HIVEP zinc finger 2 (Hs00198801_m1), purchased from Thermo Fisher Scientific, was assayed by quantitative PCR. mRNA content was normalized to 18S rRNA gene expression. Mean relative gene expression was determined by using the 2−ΔΔCt method.
ELISA
IFN-α concentration was measured on sera of non-ICU and ICU patients by using human IFN-α ELISA (EH3252, Pantec, Torino, Italy) accordingly to the manufacturer’s instructions.
Statistical analysis
A paired Student t test or one-way ANOVA test or linear regression test was applied to determine statistical significance. A p value <0.05 was considered statistically significant (*p < 0.05, **p < 0.01, ***p < 0.001). GraphPad Prism software (GraphPad Software) was used for statistical analysis.
Results and Discussion
Inflammatory cytokines produced by monocytes/macrophages play an important role in determining systemic and local damage in COVID-19. By means of CD16 and CD14 expression, we distinguished the three types of monocytes detectable in PB of SARS-CoV-2–negative HDs and COVID-19 patients admitted to our hospital, either requiring or not intensive care. As previously reported (5), nonclassical monocytes were significantly reduced in ICU patients, and an increase in classical monocytes was observed (Fig. 1A). Besides the three major subpopulations, a separate and less well-characterized monocyte subset has been previously described, which is defined by the expression of CD56 neural cell adhesion molecule. CD56+ monocytes are found in very low frequencies in PB of healthy individuals but are increased in conditions associated with autoimmunity (8). We observed an increased frequency of CD56+ monocytes in COVID-19 patients that was associated with disease severity (Fig. 1B). By ImageStream analysis we could confirm that CD56 was expressed on monocytes (Fig. 1C) and, as previously described (8), restricted to CD16− monocytes (Fig. 1D). CD56+ monocytes showed a decreased expression of HLA-DR molecules that has been previously associated with disease severity (5). Interestingly, they express high levels of CD62L, one of the major adhesion receptors supporting the rolling of leukocytes on endothelium for their extravasation into inflamed tissues. In contrast, CD16+ monocytes maintained unaltered levels of HLA-DR and CD86 costimulatory molecules and did not express CD62L (Fig. 1E). CD16+ monocytes play a critical role in the resolution of inflammation and low levels of CD16+ monocytes in SARS-CoV-2–infected patients have been reported as a marker of severity (9). In this regard, it is noteworthy that the frequency of CD56+ monocytes inversely correlated with that of CD16+ monocytes (Fig. 1F) and their ratio was significantly higher in ICU compared with non-ICU patients (Fig. 1G). RNA sequencing analysis previously revealed type I IFN–driven inflammatory features in monocytes isolated from patients with COVID-19 (10). The type I IFN system is a major antiviral defense known to also affect monocyte functions by inducing either pro- or anti-inflammatory activities. For instance, in an experimental model of influenza infection, IFN-α released by interstitial macrophages was able to suppress the proliferation of inflammatory monocyte-derived macrophages (11). Along the same line, mice with a null mutation (−/−) in the IFNAR1 gene showed increased activities of monocytes (12). However, during either SARS-CoV or MERS-CoV infections, which are more closely related to COVID-19, a delayed but considerable IFN-α response promotes, in the lungs, the accumulation of pathogenic inflammatory monocytes/macrophages and a reduction of wound-healing macrophages (13). More in general, the role of IFN-α in COVID-19 is still controversial, as either robust or poor IFN-α production has been reported in severe COVID-19 patients (14, 15). The emerging view is that IFN-α response might promote antiviral immunity during early stages of SARS-CoV-2 infection but sustained IFN-α activation during late stages could drive immunopathology of COVID-19 (16). We could confirm that culturing CD14+ monocytes in the presence of recombinant IFN-α resulted, as previously described (17), in a consistent increase of CD56 surface expression (Fig. 1H). In addition, IFN-α concentration in serum of ICU patients was higher compared with non-ICU patients (Fig. 1I) and directly correlated with the ratio of CD56+/CD16+ monocytes (Fig. 1J). Remarkably, HD monocytes cultured in the presence of ICU patients’ sera significantly upregulated CD56 expression (Fig. 1K). The induction of CD56+ monocytes induced by patients’ sera significantly decreased in the presence of neutralizing Ab for either anti IFN-α or anti IFN-αR2. (Fig. 1K, 1L). Of note, blocking IL-6, another relevant cytokine abundantly released during severe COVID-19, did not affect the frequency of CD56+ monocytes, thus emphasizing a specific contribution of IFN-α in the generation of this monocyte subset. However, because we did not observe a complete abrogation of the CD56 surface expression on monocytes upon type I IFN blocking, we cannot exclude a role of other factors in this phenomenon, including type II and type III IFNs.
FIGURE 1.
CD56+ monocytes increase in severe COVID-19 patients and are induced by IFN-α. (A) Representative dot plots and related statistical analysis show the distribution of monocyte subsets in non-ICU patients (n = 21), ICU patients (n = 38), and HDs (n = 5). Bars represent the mean percentage ± SEM of the three monocyte subsets from five independent experiments. *p < 0.05. (B) Flow cytometric analysis of CD56 expression on monocytes from non-ICU patients (n = 5), ICU patients (n = 7), and HDs (n = 3). Bars represents the mean percentage ± SEM of CD56+ monocytes from five independent experiments. ***p < 0.001. (C) ImageStream analysis shows the expression of CD14 and CD56 on monocytes from ICU patients; monocytes and NK cells from HDs were employed as control and cells defined by nuclear dye DRAQ5. (D) Expression analysis of CD56 and CD16 molecules was assessed by flow cytometry on monocytes from ICU patients. (E) Representative dot plots show the expression of CD62L and HLA-DR on CD56+ (red) and CD16+ (blue) monocytes from ICU patients (n = 4) or HDs (n = 4). Bars represent the mean percentage ± SEM of CD62L+ cells within monocyte subsets of ICU patients and HDs from four independent experiments. ***p < 0.001. (F) Correlation between the percentage of CD16+ monocytes and CD56+ monocytes in ICU patients. (G) Scatter plots indicate the ratio of CD56+/CD16+ monocytes in non-ICU (n = 21) and ICU (n = 38) patients. *p < 0.05. (H) Analysis of CD56 and CD16 expression on HD monocytes cultured 3 d with recombinant human IFN-α or left untreated. Data are representative of one experiment out of five. (I) IFN-α concentration was measured in sera of ICU (n = 8) and non-ICU (n = 8) patients by ELISA. *p < 0.05. (J) Correlation between IFN-α concentration in sera of ICU (n = 8) and non-ICU (n = 8) patients and the ratio of CD56+/CD16+ monocytes. *p < 0.05. (K) Analysis of CD56 and CD16 expression on HD monocytes cultured in the presence of serum from non-ICU or ICU patients or control serum for 3 d. One representative experiment out of three is shown; bars represent percentage ± SEM of CD56+ cells. (L) Expression of CD56 on HD monocytes cultured for 3 d with ICU serum in the presence or not of anti–IFN-α or anti–IFN-αR2 or IL-6 neutralizing mAbs was assessed by flow cytometry. Bars represent percentage ± SEM of CD56+ cells. Representative data from four independent experiments are shown. *p < 0.5, **p < 0.01. (M) Expression of PD-L1 on PB pDCs from COVID-19 patients stratified according to ratio low (n = 5) or high (n = 5) CD56+/CD16+ monocytes or HDs (n = 3) was assessed by flow cytometry. One representative experiment out of three is shown. Bars represent MFI ± SEM of PD-L1+ cells. ***p < 0.001.
Moreover, we analyzed, in COVID-19 patients, circulating pDCs, known to be main producers of IFN-α during viral infection. We observed that only pDCs derived from patients with high CD56+/CD16+ monocyte ratio expressed a high level of PD-L1 molecules, a hallmark of type I IFN–producing pDCs (18) (Fig. 1M). These results further highlighted a role for IFN-α in inducing the high frequency of CD56+ monocytes observed in severely ill patients.
Monocytes from ICU patients could produce a high level of the proinflammatory cytokine TNF-α that was abrogated in the presence of corticosteroids (Fig. 2A). Interestingly, culturing monocytes in the presence of corticosteroid resulted in a significant decrease of CD56 expression and a concomitant increase of CD16 expression that occurred even in monocytes isolated from HDs (Fig. 2B). We then analyzed the contribution of the different monocyte subsets, isolated from ICU patients, to the production of proinflammatory cytokines and observed that they were mostly produced by CD56+ monocytes upon stimulation with classical microbial TLR agonists, whereas low-to-absent production of these cytokines was detected in the CD16+ population (Fig. 2C). The predominant production of proinflammatory cytokines by CD56+ monocytes, as well as their decline upon treatment with corticosteroid, indicates a potential contribution of this cell population to the excessive inflammation observed during COVID-19. In this regard, a single-cell analysis performed in severe COVID-19 has revealed a unique monocyte subpopulation expressing ATF3, NFIL3, and HIVEP2 genes that contributes to the inflammatory status (19). We observed a significant upregulation of all of these genes in CD56+ monocytes isolated from ICU patients, indicating that CD56+ monocytes might represent the subset mainly contributing to the cytokine storm in COVID-19 (Fig. 2D). Of note, we observed that COVID-19 patients with fatal outcomes displayed a significant higher CD56+/CD16+ monocyte ratio at their entrance in the hospital (Fig. 2E).
FIGURE 2.
CD56+ monocytes show a hyperinflammatory profile and are present at a higher frequency in COVID-19 patients with fatal outcome. (A) Representative dot plots and related statistical analysis show the production of TNF-α by monocytes from HDs (n = 3) and ICU patients (n = 6) following 4 h of stimulation with LPS with or without corticosteroid. Bars represent mean percentage ± SEM of TNF-α–producing monocytes from three independent experiments. ***p < 0.001. (B) Flow cytometric analysis of CD56 and CD16 expression on monocytes isolated from HDs (n = 6) or ICU patients (n = 6) following 24 h of stimulation with corticosteroid. Bars represent the percentage of CD56+ and CD16+ monocytes in the indicated experimental conditions from three independent experiments. (C) TNF-α and IL-6 production was assessed by intracellular staining on CD56+, CD56−, and CD16+ monocytes from ICU patients following 4 h of stimulation with R848 or LPS. Dot plots are representative of one experiment out of three; bars represent mean percentage ± SEM of monocyte subsets producing TNF-α and IL-6. *p < 0.05. (D) Real-time PCR analysis of the indicated genes was assessed on CD56+ monocytes isolated from seven ICU patients and five HDs. (E) Association between CD56+/CD16+ monocyte ratio and mortality in COVID-19 patients. Scatter plots indicate the ratio of CD56+/CD16+ monocytes at hospitalization in patients with either good or fatal clinical outcome. ***p < 0.001.
Although proinflammatory monocytes from PB have been shown to infiltrate the lungs in patients with SARS-CoV-2 infection (6) and the CD56+ monocyte subset described in this study expresses a high level of CD62L (facilitating extravasation to inflamed tissues), results from this study do not provide any indication regarding the prevalence of this monocyte population within infected lung tissues. Thus, further investigation in patients’ bronchoalveolar lavage fluid or directly in inflamed tissue specimens, for example lung or endothelium obtained from autoptic samples, would be required to assess the infiltration of CD56+ monocytes in damaged tissues and allow a better understanding of their pathogenic role in severe COVID-19.
In conclusion, this study provides further insight into the complex immune dysregulation involving monocytes during COVID-19. In particular, we identified an abundant presence of CD56+ monocytes associated with the severe stage of disease. An increase of this cell population might represent, together with a decreased level of CD16+ monocytes, an inflammatory marker with negative prognostic significance in COVID-19 patients.
Our results might also have therapeutic implications in patients with severe COVID-19. Although recombinant type I IFNs have been considered for the treatment of patients with COVID-19, a retrospective study of 446 patients with COVID-19 reported that early therapeutic use of IFN-α decreased mortality, whereas late use of IFN-α increased mortality and delayed recovery (16). Thus, CD56+ monocytes may represent a type I IFN–associated biomarker useful to predict the progression to severe COVID-19 and also help to improve the clinical management of severely ill patients. Also, when the pandemic will be under control thanks to world-wide vaccination programs, the sequelae of the so-called “long COVID” and their potential burden on long-term health might still require further investigation on the role of hyperinflammatory monocyte subsets.
Footnotes
This work was supported in part by grants provided by the Italian Ministry of Health, Ricerca Finalizzata 2018 and the Italian Ministry of Education, University and Research (MIUR), PRIN 2017 (to G.F.); Finanziamento Annuale Individuale Attività Base di Ricerca FFABR by the Italian Ministry of Education, University and Research (to I.B. and P.C.); and by Roche Diagnostics (to T.P.).
S.C. designed and performed experiments, analyzed results, and wrote the manuscript; C.D.P. and G.S.M. performed experiments, analyzed data, and prepared figures; G.P. collected, stored, and processed samples; R.C., G.N., C.G., A.D., and E.V.R managed patients and provided critical biological samples and clinical parameters; T.P., I.B., and P.C. analyzed the data and critically reviewed the manuscript; and G.F. supervised the study and wrote the manuscript.
The online version of this article contains supplemental material.
Abbreviations used in this article:COVID-19
coronavirus disease 2019HD
healthy donorICU
intensive care unitPB
peripheral bloodpDC
plasmacytoid dendritic cellSARS-CoV-2
severe acute respiratory syndrome coronavirus 2