AuthorsAlexander Supadya,c,d and Robert Zeiserb Lancet Respir Med. 2022 Feb 3doi: 10.1016/S2213-2600(22)00021-2 [Epub ahead of print]PMCID: PMC8813061PMID: 35123659
Mortality is high among patients with severe COVID-19 who require invasive mechanical ventilation (IMV) or extracorporeal membrane oxygenation (ECMO).1, 2 Therefore, further specific treatment options for these patients are urgently needed.
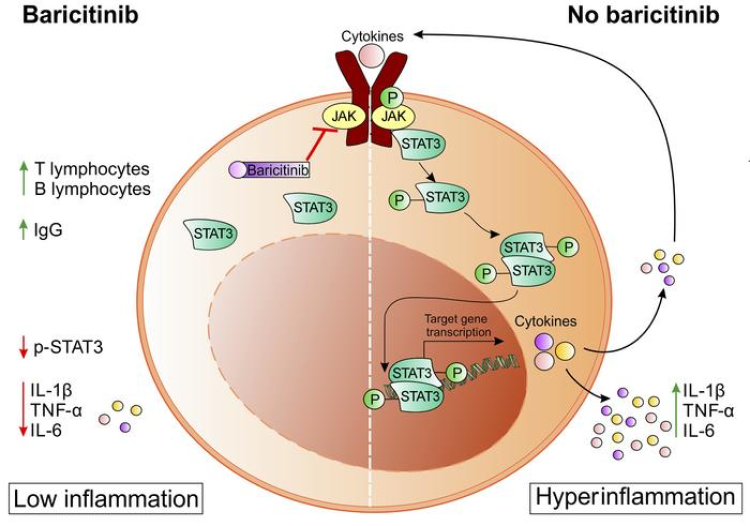
Early in the COVID-19 pandemic, Janus kinase (JAK) inhibitors were identified as potential therapeutic agents for the treatment of SARS-CoV-2 infections.3, 4 JAK inhibitors, such as ruxolitinib, fedratinib, and baricitinib, have strong anti-inflammatory properties and are already in clinical use for the treatment of graft-versus-host disease, myelofibrosis, and rheumatoid arthritis.5, 6, 7
Two large, randomised, controlled trials have assessed the use of baricitinib in hospitalised patients with COVID-19.8, 9 The Second Adaptive COVID-19 Treatment Trial (ACTT-2)8 showed that administration of baricitinib in combination with remdesivir shortened the time to recovery in hospitalised patients with COVID-19 compared with remdesivir alone. The COV-BARRIER trial9 did not show a significant benefit of baricitinib plus standard of care compared with placebo plus standard of care in the primary endpoint of disease progression by day 28. Nevertheless, although these were only secondary endpoints, 28-day mortality and 60-day mortality were significantly lower in patients who received baricitinib than in those who received placebo.
However, important questions remain, especially regarding the effect of baricitinib in patients with severe COVID-19 who require IMV or even ECMO. In the ACTT-2 trial, only 111 (11%) of 1033 patients were receiving IMV or ECMO at study inclusion; whereas in the primary COV-BARRIER trial, these severely affected patients were excluded.
In The Lancet Respiratory Medicine, E Wesley Ely and colleagues10 report the findings of an exploratory trial that followed the design of the COV-BARRIER trial and aimed to evaluate the efficacy and safety of baricitinib in addition to standard of care in critically ill hospitalised adults with COVID-19 who were receiving IMV or ECMO. Patients were randomly assigned to receive baricitinib 4 mg (n=51) or matched placebo (n=50) once daily for up to 14 days in addition to standard of care. All-cause mortality by day 28 was significantly lower in patients who received baricitinib than in those who received placebo (20 [39%] of 51 participants died in the baricitinib group vs 29 [58%] of 50 in the placebo group; hazard ratio [HR] 0·54 [95% CI 0·31–0·96]; p=0·030); this finding persisted through day 60 (23 events [45%] vs 31 [62%]; HR 0·56 [95% CI 0·33–0·97]; p=0·027). The authors concluded that baricitinib might represent a novel option for the reduction of mortality in patients with COVID-19, even in progressed disease stages (ie, when already receiving IMV or ECMO).
Although the assignment of participants to the treatment groups was randomised and masked, the mortality benefit determined in this exploratory trial should be interpreted with caution. Important baseline data for the assessment of disease severity and comparison of the study cohort with other cohorts were not provided, including duration of IMV and ECMO before study inclusion, ventilator settings, ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen, and prognostic scores, such as Sequential Organ Failure Assessment (SOFA), Simplified Acute Physiology Score (SAPS) II, or Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP). Without these supporting data, it cannot be assumed that the study groups were balanced in terms of baseline disease severity and that quality of care was consistent across study locations. Therefore, the results from this study cannot match the level of evidence from a phase 3 randomised, controlled trial with well-defined primary and secondary endpoints, accompanying sample size estimations, and a prespecified statistical analysis plan.
Interestingly, the all-cause mortality by day 60 in the placebo group in this study was high (62%), despite the baseline use of corticosteroids in 44 (88%) of 50 patients in this group.10 A meta-analysis summarising data from more than 50 000 patients with COVID-19 receiving IMV showed a mortality of only 45%, although a large number of these patients were treated without corticosteroids at the beginning of the pandemic.1 Furthermore, by contrast with the findings from this trial, in ACTT-2, in the subgroup of patients receiving IMV or ECMO at baseline, who were randomly assigned to receive baricitinib (n=54) or placebo (n=57) in addition to remdesivir but without corticosteroids, there was no significant difference between the treatment groups with respect to the outcomes of recovery time or survival by day 28.8 These observations contradict the results of the study by Ely and colleagues. At this time, we can only speculate about the reasons for these conflicting results; possible explanations are a potential influence of the concomitant treatment with remdesivir, the effect of corticosteroids, or any other differences between the groups or the treatments they received.
Taken together, we believe that the level of evidence provided by the results from this study is not sufficient to change standard of care and introduce baricitinib into clinical routine for COVID-19 patients with severe respiratory failure. However, the results of this exploratory trial and the data from COV-BARRIER and ACTT-2 reflect clinical equipoise for the addition of baricitinib to standard of care for patients with severe COVID-19 requiring IMV or ECMO, and provide a sound basis for a well-designed phase 3 trial to confirm these findings.
Open in a separate windowCopyright © 2022 Dr Barry Slaven/Science Photo Library
AS reports research grants and lecture fees from CytoSorbents and lecture fees from Abiomed, outside of the submitted work. RZ reports lecture fees from Novartis, Incyte, and Mallinckrodt, outside of the submitted work.
References
1. Lim ZJ, Subramaniam A, Ponnapa Reddy M, et al. Case fatality rates for patients with COVID-19 requiring invasive mechanical ventilation. A meta-analysis. Am J Respir Crit Care Med. 2021;203:54–66. [PMC free article] [PubMed] [Google Scholar]
2. Barbaro RP, MacLaren G, Boonstra PS, et al. Extracorporeal membrane oxygenation for COVID-19: evolving outcomes from the international Extracorporeal Life Support Organization Registry. Lancet. 2021;398:1230–1238. [PMC free article] [PubMed] [Google Scholar]
3. Richardson P, Griffin I, Tucker C, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395:e30–e31. [PMC free article] [PubMed] [Google Scholar]
4. Stebbing J, Phelan A, Griffin I, et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20:400–402. [PMC free article] [PubMed] [Google Scholar]
5. Zeiser R, von Bubnoff N, Butler J, et al. Ruxolitinib for glucocorticoid-refractory acute graft-versus-host disease. N Engl J Med. 2020;382:1800–1810. [PubMed] [Google Scholar]
6. Genovese MC, Kremer J, Zamani O, et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med. 2016;374:1243–1252. [PubMed] [Google Scholar]
7. Harrison CN, Schaap N, Vannucchi AM, et al. Janus kinase-2 inhibitor fedratinib in patients with myelofibrosis previously treated with ruxolitinib (JAKARTA-2): a single-arm, open-label, non-randomised, phase 2, multicentre study. Lancet Haematol. 2017;4:e317–e324. [PMC free article] [PubMed] [Google Scholar]
8. Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med. 2021;384:795–807. [PMC free article] [PubMed] [Google Scholar]
9. Marconi VC, Ramanan AV, de Bono S, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir Med. 2021;9:1407–1418. [PMC free article] [PubMed] [Google Scholar]
10. Ely EW, Ramanan AV, Kartman CE, et al. Efficacy and safety of baricitinib plus standard of care for the treatment of critically ill hospitalised adults with COVID-19 on invasive mechanical ventilation or extracorporeal membrane oxygenation: an exploratory, phase 3, randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2022 doi: 10.1016/S2213-2600(22)00006-6. published online Feb 3. [CrossRef] [Google Scholar]