Authors: Patrick J Lindsay, a Rachel Rosovsky, b Edward A Bittner, c and Marvin G. Chang c
ABSTRACT
Introduction
COVID-19-associated coagulopathy (CAC) is a well-recognized hematologic complication among patients with severe COVID-19 disease, where macro- and micro-thrombosis can lead to multiorgan injury and failure. Major societal guidelines that have published on the management of CAC are based on consensus of expert opinion, with the current evidence available. As a result of limited studies, there are many clinical scenarios that are yet to be addressed, with expert opinion varying on a number of important clinical issues regarding CAC management.
Methods
In this review, we utilize current societal guidelines to provide a framework for practitioners in managing their patients with CAC. We have also provided three clinical scenarios that implement important principles of anticoagulation in patients with COVID-19.
Conclusion
Overall, decisions should be made on a case by cases basis and based on the providers understanding of each patient’s medical history, clinical course and perceived risk.
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first identified in December 2019 in Wuhan, China. The disease was initially identified as a cluster of pneumonia cases; however, the disease dispersed rapidly and was formally declared a pandemic by the World Health Organization (WHO) in March 2020. As of June 2021, there have been greater than 172 million cases reported worldwide, including more than 3.7 million deaths [1]. The initial manifestations of SARS-CoV-2 vary, and usually occur between one and 14 days from exposure to the virus. Risk factors identified for COVID-19 include male gender, obesity, cardiovascular disease, diabetes mellitus, and older age [2–5]. The most common initial symptoms include fever, cough, fatigue, and loss of smell or taste [6]. During this period, the virus infects the epithelial cells through the angiotensin-converting enzyme 2 receptors and may eventually present as a viral pneumonia.
Although the disease primarily affects the respiratory system, multi-system organ involvement can occur with increasing severity of disease [6–8]. COVID-19-associated coagulopathy (CAC) is a well-recognized hematologic complication among patients with severe COVID-19 disease, where macro- and micro-thrombosis can lead to multiorgan injury and failure [7,9]. This identification has led to significant clinical questions regarding the optimal prevention and management of CAC, which in turn, has led to many ongoing clinical trials. To help guide the management of these patients in the interim, numerous major societies have put forth recommendations regarding diagnosis, monitoring, and treatment of CAC. In this report, we discuss the pathogenesis, prevalence, diagnosis, and treatment of CAC to help providers understand this complicated condition and to apply best practices to the care of their patients.
From a practical perspective, societal guidelines are based on consensus of expert opinion, with the current evidence available. Major societal guidelines that have published on the management of CAC include but are not limited to Centers for Disease Control and Prevention (CDC), International Society on Thrombosis and Hemostasis interim guidance (ISTH-IG), American Society of Hematology (ASH), American College of Chest Physicians (ACCP), Scientific and Standardization Committee of ISTH (SCC-ISTH), Anticoagulation Forum (ACF), and American College of Cardiology (ACC) [9–13]. As a result of limited studies, there are many clinical scenarios that are yet to be addressed, with expert opinion varying on a number of important clinical issues regarding CAC management. In this review, we utilize current societal guidelines to provide a framework for practitioners in managing their patients with CAC. To supplement the manuscript, we have provided three clinical scenarios (supplemental material) which implement important principles of anticoagulation in patients with COVID-19. Overall, decisions should be made on a case by cases basis and based on the providers understanding of each patient’s medical history, clinical course and perceived risk.
Prevalence
There is an increasing body of evidence suggesting patients with COVID-19 demonstrate a higher incidence of thromboembolic disease compared to historical data [14,15], with patients admitted to the ICU being at highest risk [16,17]. The true incidence of thromboembolism in patients admitted to hospital remains controversial with rates as low as 1% in those admitted to the medical ward and up to 69% of patients in the ICU [5,18]. In one study of 107 ICU patients, 91% of whom received VTE prophylaxis and 9% who received therapeutic anticoagulation, the prevalence of PE was 20.4%. In comparison to patients admitted to ICU for other reasons, patients with COVID-19 were 3–4 fold more likely to develop a pulmonary embolism (PE) [19]. However, there is also data to suggest that the prevalence of VTE is similar to that of patients admitted to hospital with similar non COVID illnesses, of similar severity [20,21]. The reported increase in prevalence of VTE remains despite thromboprophylaxis in some studies and not in others [15,22]. In addition to macro-vascular thrombosis, autopsy studies have demonstrated significant microvascular thrombosis in the lungs of patients who have died from COVID-19’s [7,23]. It is hypothesized that these microvascular thrombi cause end organ dysfunction such as renal failure [7,23,24]. Although the prevalence of microvascular thrombosis is yet to be determined, it may be greater than in patients with non COVID respiratory viral illnesses [24].
The majority of data on the prevalence of thromboembolic disease in patients with COVID-19 have been observational studies of VTE. However, there is emerging evidence suggesting an increase in arterial thromboses in COVID-19 patients as well. One study identified five cases of acute ischemic stroke in a two-week period in COVID-19 patients under the age of 50, in comparison to 0.7 large vessel strokes per two-weeks prior to the pandemic [25]. There are also studies reporting an increase in the prevalence of acute limb ischemia in patients hospitalized with COVID-19 compared to the general population [26,27].
The micro- and macro-thrombosis associated with CAC often leads to multisystem complications resulting in increased morbidity and mortality [8]. Despite many policies aimed at curbing viral spread, many countries/regions still see their weekly ICU admissions related to COVID-19 increasing [1]. With the introduction of vaccinations and targeted novel treatments for COVID-19, the prevalence and incidence of CAC and its related complications will hopefully decrease.
Pathogenesis
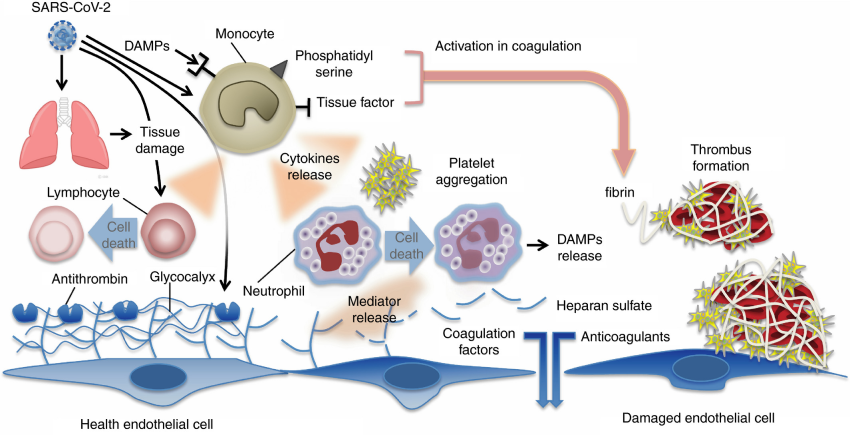
Although the pathogenesis of CAC has not been fully elucidated, there are multiple contributing factors that include hypercoagulability, endothelial dysfunction and abnormal blood flow (especially in the pulmonary vasculature) [28]. In severe COVID-19 disease, excess proinflammatory cytokines trigger the coagulation system resulting in a hypercoagulable state [29]. In addition to direct damage by viral invasion, this cytokine storm also results in endothelial injury and dysfunction, leading to endothelial cell activation [30]. The dysfunctional endothelial cells produce excess thrombin as well as shutdown fibrinolysis, leading to a prothrombotic state [31]. Furthermore, infection induced inflammatory changes in endothelial cells have been shown to increase coagulation biomarkers, including factor VIII, von Willebrand Factor (vWF), fibrinogen and P-selectin [24,32]. All of these mechanisms create an imbalance in the normal hemostatic system, with a resulting prothrombotic state, manifesting as both macro and micro-vascular thrombosis [30].
In patients with CAC, biomarkers supporting the hypercoagulability pathogenesis have been demonstrated to be elevated in vivo. Specifically, the procoagulant markers Factor VIII, Von Willebrand antigen and Von Willebrand activity have been found to be markedly elevated in patients with COVID-19 who develop thromboembolism [33]. Biochemically, this hypercoagulable state appears to be most significant in those patients with the most severe form of the disease. One study demonstrated that many prothrombotic markers are increased above their upper limit of normal in patients with COVID-19, with the greatest increase in patients admitted to the intensive care unit (ICU) as compared to non-ICU patients [34]. In support of this recognition, descriptive studies using viscoelastic hemostasis assays including thromboelastography (TEG) and rotational thromboelastometry (ROTEM) in patients with COVID-19 are more consistent with a hypercoagulable state rather than acute disseminated intravascular coagulation (DIC). For example, in a study of 24 ICU patients with COVID-19, TEG parameters demonstrated a hypercoagulable state with decreased K (kinetic) time (increased fibrinogen activity), increased alpha angle (increased fibrinogen activity), increased maximum amplitude (Ma) (increased platelet activity), and decreased LY30 (decreased fibrinolysis) [33]. These findings suggest decreased time to clot accumulation, increased strength and stability of the clot, and decreased breakdown of the clot. This study also found that fibrinogen, D-dimer, C-reactive protein, Factor VIII, vWF and protein C were all increased, while platelet count was normal or increased, prothrombin time (PT) and partial thromboplastin time (PTT) were near normal and antithrombin (AT) was marginally decreased. These discoveries are all consistent with a hypercoagulable state rather than DIC where one would expect decreased platelet count and increased PT and PTT. In another study of 21 ICU patients with COVID-19, the TEG MA was significantly higher in those with high thrombotic events (≥2 thrombotic events, defined as an arterial, central venous, or dialysis catheter or filter thromboses) compared to those with low thrombotic events (0–1 thrombotic events) [35]. Moreover, the mean fibrinogen and D-dimer levels were elevated in those with high thrombotic events although there was no significant differences in PT, international normalized ratio (INR), PTT or platelets between the two groups.
D-dimer elevation has also been associated with severity of COVID-19 and may be useful as a prognostic marker [36]. D-dimers are fibrin degradation product which can be measured in the blood after a clot is broken down through fibrinolysis [37]. As a result, elevated D-dimer levels are suggestive of venous thromboembolism (VTE), pulmonary embolism (PE) or DIC; however, it is also elevated in the context of systemic inflammation, which has been demonstrated in numerous clinical settings [20,38]. With COVID-19 stimulating a systemic inflammatory response, it is likely that D-dimers are elevated whether thromboembolic disease is present or not.
Despite the increased inflammatory response associated with COVID-19, patients are less likely to develop a reactive thrombocytosis and often have a mild thrombocytopenia [39]. Platelets in patients with COVID-19 have been found to have increased activity compared to healthy patients, with increased aggregation, thromboxane generation and platelet activation [40]. It is unclear whether these changes in platelet function are associated with an increased risk of thrombosis [39,41]. One study in hospitalized patients with COVID-19 found that a platelet count >450 × 109/L on admission was associated with an increased risk of VTE (adjusted OR of 3.56 [95% CI, 1.27–9.97]) [20]. However, another study of 1476 patients hospitalized with COVID-19 found that the platelet count was inversely associated with risk of in-hospital mortality, although, it is unclear if this increased risk in mortality was a result of consumption in the context of DIC [42]. The appropriate workup of a decreasing platelet count and thrombocytopenia should be pursued in the COVID-19 patient as it would be for non-COVID patients. Given that the majority of patients with COVID-19 receive some form of heparin, a diagnosis of heparin-induced thrombocytopenia (HIT) may be considered, and the subsequent use of Platelet Factor 4 (PF4) and platelet serotonin release assay (SRA) can be used to diagnose this potentially life-threatening condition.
Upon initial screening, many patients with COVID-19 have an elevated PTT in the context of minimal clinical bleeding. This finding raises the question of whether the thrombotic related biomarker, lupus anticoagulant is affected by COVID-19 disease [43,44]. The lupus anticoagulant is an immunoglobulin which binds to phospholipids and proteins associated with the cell membrane. When evaluated in vitro, these immunoglobulins interfere with the phospholipids which induce coagulation, leading to a prolonged PTT. In vivo, however, these antibodies are often prothrombotic [45]. Two studies of patients with COVID-19 and prolonged PTT on initial presentation have demonstrated high rates of lupus anticoagulant positivity [15,43]. However, it has been noted that upon repeat testing, many patients become negative, suggesting that the lupus anticoagulant positivity may be transient and associated with severe viral illness [46]. Overall, more studies are needed to determine if lupus anticoagulant is truly associated with COVID-19 and related to an increase in VTE, and if so, whether the use of anticoagulation should be changed based on the presence of this abnormality [47].
Antithrombin deficiency, a marker of thrombophilia, can be acquired or inherited and may place patients at increased risk of thrombosis. Antithrombin is a natural anticoagulant that inhibits thrombin and factor Xa, thereby helping to prevent thrombosis. There have been case reports of AT deficiency in COVID-19 patients which may place such patients are at higher risk of thrombosis [48]. However, as with the other biochemical testing, more studies are needed to determine if this knowledge is related to adverse outcomes such as thrombosis and if these results should be used to help guide anticoagulation practices.
Manifestations
Since the emergence of COVID-19, the associated increased risk of thrombotic complications such as deep vein thrombosis (DVT), pulmonary embolism (PE) and microvascular thrombosis have been well described [17]. The spectrum of CAC is broad and may present with either or both arterial and venous thromboembolic disease [22]. In general, the majority of the thrombotic events when present are DVT or PE, however catheter associated thrombosis, other venous thrombosis and arterial events, including stroke, acute limb ischemia, bowel ischemia and myocardial infarction, have been reported [14]. Patients also appear to be at risk for microvascular thrombosis. Multiple autopsy studies have demonstrated microvascular thrombosis in the lungs of patients who have died from COVID-19’s, suggesting the multisystem organ failure often seen in COVID-19 may be a result of microvascular thrombosis [23,49].
Bleeding was initially believed to be much less common than thrombosis in patients with COVID-19, however, further data has emerged suggesting the rates of bleeding may be similar to the rates of thrombosis [20]. One autopsy study involving 82 patients found that, 6% died from hemorrhage and that over 80% of patients had some kind of hemorrhagic complication [50]. Another study of 400 hospitalized COVID-19 patients found a radiographically confirmed VTE rate of 4.8% (95% confidence interval [CI], 2.9–7.3), which was identical to the overall bleeding rate of 4.8% (95% CI, 2.9–7.3) [20].
Although clinically significant DIC is uncommon in COVID-19 patients, and if suspected, it is important to identify and aggressively treat the underlying etiology, including any superimposed bacterial infections in addition to managing the coagulopathy [51].
Diagnosis
Currently, there are no diagnostic criteria for CAC. At present, there is no evidence or guideline to support routine screening for VTE, PE or other thrombotic complications in patients with COVID-19. However, the threshold to investigate for DVT or PE should be low given the frequency with which these complications may occur in patients with COVID-19. If thromboembolic disease is suspected, appropriate investigations should depend on the clinical context, acuity of disease and resources available. A position paper from the National Pulmonary Embolism Response Team (PERT) Consortium provides a step wise approach to a suspected PE, which includes ordering a computed tomography pulmonary angiogram (CTPA) if available [52]. If the computed tomography (CT) is not available or the patient is too unstable, a lower limb ultrasound to assess for a proximal DVT, or an echocardiography to assess right heart strain may be pursued. However, it should be noted that neither of these investigations are sensitive. If none of those modalities are available, nor do they rule in a PE/DVT, and there is a high clinical suspicion for a PE, therapeutic anticoagulation should be considered pending no absolute contraindications [52].
In patients with COVID-19, many routine biomarkers of the coagulation cascade have been found to fall outside the normal range, including PTT/PT, platelet count, and fibrinogen [53]. Derangement of these tests may suggest increased disease severity. A D-dimer elevated by three- to fourfold, a prolonged prothrombin time and a platelet count <100 × 109 are all predictors of a poorer prognosis [13,54]. Despite this evidence, most of the major societal guidelines (ACF, ACCP, SCC-ISTH, CDC) have either not recommended nor commented on the routine monitoring of laboratory values to guide management, risk stratification, or triage of patients with COVID-19. For instance, the SCC-ISTH guidelines state that further study is required before using laboratory testing for risk stratification and triage of CAC. The CDC guidelines state that there is a lack of prospective data demonstrating laboratory testing as a way to risk stratify patients, and that there are insufficient data to recommend for or against using laboratory values to guide management. If a patient is bleeding or has a confirmed, or is highly suspected of having a VTE; then, the patient should be treated based on clinical context. Repeat testing of CBC, coagulation studies, fibrinogen, and d-dimer should also be performed based on the clinical setting.
When considering the use of D-dimer assays, a negative D-dimer can be useful in excluding a VTE in patients with COVID-19. However, a positive D-dimer does not necessarily equate to a diagnosis of VTE as this test is not specific and can be elevated in many other pathological and non-pathological processes [55]. Moreover, studies have demonstrated that D-dimer levels tend to be higher in severe COVID-19 cases and may be used as a potential prognostic marker [56]. Despite this connection, none of the major societal guidelines recommend routine monitoring of D-dimer nor using it to guide anticoagulation practices.
Treatment
At least seven major societal guidelines have been published to address prevention and treatment of CAC in the critical care settings, with all authors of these guidelines having prior expertise in the management of VTE [57]. Some of the societies with published guidelines include Centers for Disease Control and Prevention (CDC), International Society on Thrombosis and Hemostasis interim guidance (ISTH-IG), American Society of Hematology (ASH), American College of Chest Physicians (ACCP), Scientific and Standardization Committee of ISTH (SCC-ISTH), Anticoagulation Forum (ACF), and American College of Cardiology (ACC) [9–13]. Currently, there are no separate recommendations for prevention or management of arterial thrombosis in CAC; therefore, this patient population should follow the recommendations of the clinical syndrome in question (e.g., acute myocardial infarction requires dual antiplatelets).
Here, we review the recommendations for prevention, treatment, and monitoring of anticoagulation for CAC in the critical care setting by reviewing the common questions. A summary of the recommendations can be seen in Table 1, Table 2 and Table 3:
- How should biomarkers be used to guide management?
- What are the preferred prophylactic anticoagulation regimens?
- When should the intensity of anticoagulation be increased?
- What are the preferred therapeutic anticoagulation regimens?
- When are thrombolytics recommended?
- When should anticoagulation be held?
- What is the utility of mechanical thromboprophylaxis?
- What is the appropriate method of monitoring anticoagulation?
- What is the recommended approach for correction of active bleeding?
- Should patients receive post-discharge prophylactic anticoagulation and if so, what regimens are recommended?
Table 1.
Major societal recommendations regarding using biomarkers to guide anticoagulation, choice of prophylactic anticoagulation and when to consider increasing intensity of anticoagulation
| How should biomarkers be used to guide management? | What are the preferred prophylactic anticoagulation regimens? | When should the intensity of anticoagulation be increased? | |
|---|---|---|---|
| CDC | Insufficient data to recommend for or against using hematologic and coagulation parameters to guide management decisions. | LMWH or UFH (standard dosing). Insufficient data to recommend for or against the increase of anticoagulation intensity outside of a clinical trial. | Consider when a clinically suspected thromboembolic event is present or highly suspected despite imaging confirmation. Insufficient data to recommend for or against the increase of anticoagulation intensity outside the context of a clinical trial. Mentions patients who have thrombosis of catheters or extracorporeal filters should be treated accordingly to standard institutional protocols for patients without COVID-19. |
| ISTH-IG | Not mentioned | LMWH (standard dosing) | No specific recommendations |
| ACF | Biomarker thresholds such as D-dimer for guiding anticoagulation management should not be done outside the setting of a clinical trial. | Suggests an increased intensity of venous thromboprophylaxis be considered for critically ill patients# (i.e. LMWH 40 mg SC twice daily, LMWH 0.5 mg/kg subcutaneous twice daily, heparin 7500 SC three times daily, or low-intensity heparin infusion) that they state is based largely on expert opinion. | Consider when a clinically suspected thromboembolic event is present or highly suspected despite imaging confirmation. |
| ASH | No particular change to regimen recommended for patients with lupus like inhibitors. TEG and ROTEM should not be used routinely to guide management. | LMWH over UFH (standard dosing) to reduce exposure unless risk of bleeding outweighs risk of thrombosis. | Consider increasing the intensity of anticoagulation regimen (i.e. from standard to intermediate intensity, from intermediate to therapeutic intensity) or change anticoagulants in patients who have recurrent thrombosis of catheters and extracorporeal circuits (i.e. ECMO, CRRT) on prophylactic anticoagulation regimens. |
| ACCP | Not mentioned | LMWH (standard dosing) | Patients with PE or proximal DVT. |
| SCC-ISTH | D-dimer levels should not be used solely to guide anticoagulation regimens. | LMWH or UFH. Intermediate intensity LMWH can be considered in high risk critically ill patients (50% of responders) and may be considered in non-critically ill hospitalized patients (30% of respondents). Mentions that there are several advantages of LMWH over UFH including once vs twice or more injections and less heparin-induced thrombocytopenia. Regimens may be modified based on extremes of body weight (50% increase in dose if obese), severe thrombocytopenia*, or worsening renal function. | Therapeutic anticoagulation should not be considered for primary prevention until randomized controlled trials are available. Increased intensity of anticoagulation regimen (i.e. from standard or intermediate intensity to therapeutic intensity) can be considered in patients without confirmed VTE or PE but have deteriorating pulmonary status or ARDS. |
| ACC | Further investigation is required to determine the role of antiphospholipid antibodies in pathophysiology of COVID-19- associated thrombosis. D-dimer > 2 times the upper limit may suggest that patient is at high risk for VTE and consideration of extended prophylaxis (up to 45 days) in patients at low risk of bleeding. | Mentions that therapeutic anticoagulation is the key to VTE treatment. Does not make distinction between confirmed or suspected VTE. Hemodynamically stable patients with submassive PE should receive anticoagulation rather than thrombolytics |
Table 2.
Major societal recommendations regarding therapeutic anticoagulation regimens, when thrombolytics should be used and when anticoagulation should be held
| What are the preferred therapeutic anticoagulation regimens? | When should anticoagulation be held? | When are thrombolytics recommended? | |
|---|---|---|---|
| CDC | Standard regimens for non-COVID-19 patients. | Active hemorrhage or severe thrombocytopenia (Platelet count not defined) | Insufficient data to recommend for or against thrombolytic therapy outside the context of a clinical trial. In pregnant patients, thrombolytic therapy should only be used for acute PE with life-threatening hemodynamic instability due to risk for maternal hemorrhage. |
| ISTH-IG | Not mentioned | Hold when signs of active bleeding or platelet count < 25 x 109/L. Abnormal PT or PTT is not a contraindication to thromboprophylaxis. | Not mentioned |
| ACF | LMWH over UFH whenever possible to avoid additional laboratory monitoring, exposure, and personal protective equipment. In patients with AKI or creatinine clearance < 15–30 mL/min, UFH is recommended over LMWH. | Active bleeding or profound thrombocytopenia (Platelet count not defined) | Consider if clinical indication such as STEMI, acute ischemic stroke, or high-risk massive PE with hemodynamic instability. Otherwise, it is not recommended outside context of a clinical trial. |
| ASH | LMWH or UFH over direct oral anticoagulants due to reduced drug-drug interactions and shorter half-life. | Thromboprophylaxis is recommended even with abnormal coagulation tests in the absence of active bleeding and held only if platelet count < 25 x 109/L or fibrinogen < 0.5 g/L. Abnormal PT or PTT is not a contraindication to thromboprophylaxis. Therapeutic anticoagulation may need to be held if platelet count < 30–50 x 109/L or fibrinogen < 1.0 g/L. | Not mentioned |
| ACCP | LMWH or fondaparinux over UFH. UFH preferred in patients at high bleeding risk and in renal failure or needing imminent procedures. Recommend increasing dose of LMWH by 25–30% in patients with recurrent VTE despite therapeutic LMWH anticoagulation. | Not mentioned | Thrombolytics over no such therapy in patients with objectively confirmed PE with hemodynamic instability or signs of obstructive shock who are not at high risk of bleeding. Peripheral thrombolysis recommended over catheter-directed thrombolysis |
| SCC-ISTH | Not mentioned | No specific recommendations. Reports that 50% of respondents report holding if platelet count < 25 x 109/L. | Not mentioned |
| ACC | Medication regimen likely to change depending on comorbidities (i.e. renal or hepatic dysfunction, gastrointestinal function, thrombocytopenia). Parenteral anticoagulation (i.e. UFH) may be preferred in many ill patients given it may be withheld temporarily and has no known drug-drug interactions with COVID-19 therapies. LMWH may be preferred in patients who are unlikely to need procedures as there are concerns with UFH regarding the time to achieve therapeutic PTT and increased exposure to healthcare workers. DOACs have advantages including lack of monitoring that is ideal for outpatient management but may have risks in settings of organ dysfunction related to clinical deterioration and lack of timely reversal at some centers. | In patients with moderate or severe COVID-19 on chronic therapeutic anticoagulation who develop suspected or confirmed DIC without overt bleeding, it is reasonable to consider the indication of anticoagulation and risk of bleeding for adjusting dose or discontinuation of anticoagulation. The majority of authors recommended reducing the intensity of anticoagulation unless there was an exceedingly high risk of thrombosis. | A multidisciplinary PERT may be helpful for intermediate and high-risk patient with VTE. For hemodynamically high-risk PE, systemic fibrinolysis is indicated with catheter-based therapies reserved for situations that are not amenable to systemic fibrinolysis. Patients with hemodynamically stable intermediate-low or intermediate-high risk PE should receive anticoagulation and rescue systemic fibrinolysis should be considered in cases of further deterioration with catheter-directed therapies as an alternative. Catheter directed therapies should be limited to most critical situations given minimal data showing mortality benefit. When considering fibrinolysis vs percutaneous coronary intervention for STEMI, clinicians should weigh risks and severity of STEMI presentation, severity of COVID-19 in patient, risk of COVID-19 to individual clinicians and healthcare system. |
Table 3.
Major societal recommendations regarding monitoring of anticoagulation, correction of active bleeding and prophylactic anticoagulation post-discharge
| What is the appropriate method of monitoring anticoagulation? | What is the recommended approach for correction of active bleeding? | Should patients receive post-discharge prophylactic anticoagulation | |
|---|---|---|---|
| CDC | Per standard of care for patients without COVID-19 | Not mentioned | Routine venous thromboprophylaxis post-discharge is not recommended. FDA-approved prophylactic anticoagulation regimen (rivaroxaban and betrixaban) can be considered if high risk for VTE and low risk for bleeding using criteria from clinical trials. |
| ISTH-IG | Not mentioned | Transfuse to keep platelet count > 50 x 109/L, fibrinogen > 1.5 g/L, PT ratio < 1.5 | No specific recommendations |
| ACF | Recommend monitoring anti-Xa levels to monitor UFH due to potential baseline PTT abnormalities. Reasonable to monitor anti-Xa or PTT in patients with normal baseline PTT levels and do not exhibit heparin resistance (> 35,000 u heparin over 24 h). | Not mentioned | No evidence for anticoagulation beyond hospitalization, but reasonable to consider if low risk for bleeding and high risk for VTE including intubated, sedated, and paralyzed for multiple days. |
| ASH | May necessitate anti-Xa monitoring of UFH given artefactual increases in PTT. | Transfuse one adult unit of platelets if platelets < 50 x 109/L, give 4 units of plasma if INR > 1.8, and fibrinogen concentrate (4 g) or cryoprecipitate (10 u) if fibrinogen < 1.5 g/L. In patients with severe coagulopathy and bleeding can consider 4 F-PCC (25 u/kg) instead of plasma. | Reasonable to consider FDA-approved post-discharge prophylactic anticoagulation regimen (rivaroxaban and betrixaban) or aspirin if criteria from trials for post-discharge thromboprophylaxis are met. |
| ACCP | Monitor anti-Xa levels in all patients receiving UFH given potential of heparin resistance. | Not mentioned | Can be considered in patients who are at low risk of bleeding if emerging data suggests a clinical benefit. |
| SCC-ISTH | No specific recommendations. Mentions that expert clinical guidance statements and clinical pathways from large academic healthcare systems target an anti-factor Xa level of 0.3–0.7 IU/mL for UFH. | Not mentioned | Either LMWH or FDA-approved post-discharge prophylactic anticoagulation regimen (rivaroxaban and betrixaban) should be considered in patients with high VTE risk criteria. Duration is 14 days at least and up to 30 days. Of note, they report that none of the respondents recommended aspirin for post-discharge thromboprophylaxis. |
| ACC | Not mentioned | Transfuse platelets to maintain platelets > 50 x 109/L in DIC and active bleeding or if platelets < 20 x 109/L in patients at high risk of bleeding or requiring invasive procedures. FFP (15 to 25 mL/kg) in patients with active bleeding with either prolonged PT or PTT ratios (> 1.5 times normal) or decreased fibrinogen (< 1.5 g/L). Fibrinogen concentrate or cryoprecipitate in patients with persisting severe hypofibrinogenemia (< 1.5 g/L). Prothrombin complex concentrate if FFP is not possible. Tranexamic acid should not be used routinely in patients with COVID-19-associated DIC given the existing data. | Reasonable to consider extended prophylaxis with LMWH or DOACs for up to 45 days in patients at high risk for VTE (i.e. D-dimer > 2 times the upper limit, reduced mobility, active cancer) and low risk of bleeding. |
How should biomarkers be used to guide management?
Despite CAC being associated with biomarker abnormalities, none of the major societies to date recommend the use of biomarkers to help guide inpatient anticoagulation decisions. Most notably, D-dimer elevation has been associated with severity of COVID-19, and may be useful as a prognostic marker [36]. With COVID-19 triggering a systemic inflammatory response, it is likely D-dimers will be elevated whether thromboembolic disease is present or not. Therefore, none of the major societies recommend any role for routine monitoring of D-dimer, with its use limited to risk stratification as per ISTH-IG and ASH. Furthermore, the CDC guidelines specifically state that there is insufficient data to recommend for or against using hematologic and coagulation parameters to guide management decisions in CAC. The ACF guidelines also states that using biomarkers such as D-dimer for guiding anticoagulation management should only be done in the setting of a clinical trial. One society’s guidelines, the ACC, mentions a potential role for the use of biomarkers in decision-making: in patients with a D-dimer >2 times the upper limit may be considered for extended prophylaxis (up to 45 days) if patients are at low risk of bleeding. Currently, a multicenter randomized controlled trial is underway to evaluate the efficacy and safety of antithrombotic strategies in COVID-19 adults not requiring hospitalization at time of diagnosis. The trial is designed to compare aspirin, low dose and regular dose apixaban prophylaxis and placebo, with the results of VTE compared across increasing D-dimer levels [58]. This study will help ascertain the value of baseline D-dimer levels in this population. Another clinical trial, ATTACC, was performed to determine whether therapeutic anticoagulation improved organ support-free days [59]. This study also assessed the efficacy of therapeutic anticoagulation across subgroups based on initial D-dimer level. The D-dimer level did not appear to be useful in risk stratification.
When considering other biomarkers, none of the major societal guidelines recommend a change to the anticoagulation regimen in patients with COVID19 who have positive antiphospholipid antibodies or any other biomarker abnormality. Finally, although viscoelastic hemostasis assays such as TEG and ROTEM may suggest hypercoagulability, the ASH guidelines specifically recommend against the routine use of these tests to guide management.
What are the preferred prophylactic anticoagulation regimens?
VTE prophylaxis should be provided to all hospitalized patients with COVID-19 unless contraindicated. The majority of societal guidelines have recommended once daily administration using low molecular weight heparin (LMWH) to reduce healthcare worker exposure given the lower frequency of administration compared to unfractionated heparin (UFH), to conserve personal protective equipment and because it has a lower risk for heparin-induced thrombocytopenia. LMWH may not be preferred over UFH when the risk of bleeding outweighs the risk of thrombosis and in patients with renal dysfunction (i.e. creatinine clearance <30 mL/min). An additional benefit of heparin is its possible anti-inflammatory effects in both the vasculature and the airway [59]. With COVID-19 stimulating a proinflammatory state in both the airways and vasculature, heparin not only provides value as an anticoagulant but also may exert benefit as an anti-inflammatory agent [60]. The efficacy of heparin as an anti-inflammatory agent in patients with COVID-19 warrants further investigation.
The dosing of prophylactic anticoagulation remains controversial given that some studies have demonstrated that up to one quarter of patients with COVID in the ICU develop VTE despite thromboprophylaxis [17,22,61]. As a result, it has been suggested that intermediate or therapeutic doses of LMWH could be considered [10,12,62,63]. There is emerging evidence that initiation of therapeutically dosed anticoagulation in place of prophylactically dosed anticoagulation may decrease the need for mechanical ventilation and other life supporting interventions in non-critically ill hospitalized population but this has not been published in a peer review journal [59]. With thrombosis being a prominent feature of COVID-19, three clinical trials conducted a multiplatform clinical trial (ATTACC) to determine whether therapeutic anticoagulation improved organ support-free days (ICU level care and receipt of mechanical ventilation, vasopressors, extracorporeal membrane oxygenation (ECMO) or high-flow nasal oxygen). Although full results have not been published, the pre-publication, non-peer-reviewed, interim results show that patients who are moderately ill (hospitalized but not on ICU organ-support) had improved organ support-free days with therapeutically dosed anticoagulation in comparison to standard of care [64]. However, full-dose anticoagulation when started in critically ill patients with COVID19 was not found to be beneficial and may be harmful. Current societal guidelines which do not account for these interim findings do not recommend the use of therapeutically dosed anticoagulation as a replacement for prophylactically dosed anticoagulation. Until the results of these studies are published and validated, following current guidelines seems reasonable. Of note, ASH published new guidelines in March 2021 which continue to recommend prophylactically dosed anticoagulation in the context of hospitalized patients diagnosed with COVID-19 [65].
A retrospective analysis of 4389 COVID-19 patients found that compared with no anticoagulation, therapeutic and prophylactic anticoagulation were associated with a lower in-hospital mortality and intubation. Furthermore, when anticoagulation was initiated ≤48 h from admission, there was no statistically significant difference in outcomes between the patients that received therapeutic vs. prophylactic doses [66].
Finally, aspirin has been a proposed treatment for CAC given its anti-inflammatory and anti-thrombotic effects [67–70]. A recent meta-analysis demonstrated no association between the use of aspirin and mortality in COVID-19 [71]. The RECOVERY trial conducted a multicentre randomized control trial (RCT) testing aspirin against usual care [72]. The results of this trial released in preprint showed that aspirin was not associated with a reduction in 28-day mortality or in risk of progressing to invasive mechanical ventilation or death.
When to increase intensity of anticoagulation
At this stage, there is no consensus as to when to increase the intensity of anticoagulation with the exception of documented thromboembolism. The ACF states that increased intensity of anticoagulation regimen (i.e., from standard or intermediate intensity to therapeutic intensity) can be considered in patients, without confirmed VTE or PE, who have deteriorating pulmonary function or ARDS without clear underlying cause. They also suggest an increased intensity of venous thromboprophylaxis could be considered for critically ill patients (i.e. LMWH 40 mg SC twice daily, LMWH 0.5 mg/kg subcutaneous twice daily, heparin 7500 SC three times daily, or low-intensity heparin infusion). The SCC-ISTH guidelines state that intermediate intensity LMWH may be considered in high risk critically ill patients. They also suggest anticoagulation prophylaxis regimens may be modified based on extremes of body weight. If the patient is obese (BMI >30 kg/m2), an increase of 50% in dose has been deemed reasonable.
ASH guidelines state that in patients who have recurrent thrombosis of catheters and extracorporeal circuits (i.e., ECMO, continuous renal replacement therapy (CRRT)) on prophylactic anticoagulation regimens, may have the intensity of anticoagulation increased (i.e. from standard to intermediate intensity, from intermediate to therapeutic intensity) or change the anticoagulant regimen. The CDC guidelines state that patients who have thrombosis of catheters or extracorporeal filters should be treated according to standard institutional protocols (which may include increasing anticoagulation intensity) for patients without COVID-19. Our institution, the Massachusetts General Hospital found that a low dose heparinized saline protocol is associated with improved duration of arterial line patency in critically ill COVID-19 patients [73]. Additionally, we also found that a protocol where systemic unfractionated heparin is dosed by anti-factor Xa levels lead to lower rates of CRRT filter clotting and loss [74].
What is the preferred therapeutic anticoagulation regimens?
Several of societal guidelines (ACF, ACCP, and ACC) recommend LMWH over UFH to avoid additional laboratory monitoring, minimize healthcare worker exposure, preserve personal protective equipment (PPE) utilization, benefit from the greater anti-inflammatory effects, and decrease time to achieve therapeutic anticoagulation levels. LMWH is preferred over UFH when no imminent procedures are planned, the risk of thrombosis is greater than the risk of bleeding, and patients do not have significant renal failure. In addition to LMWH, the ACCP guidelines also recommend fondaparinux over UFH with a similar rationale, and fondaparinux may be used in patients with suspected or confirmed HIT. UFH may be preferred in patients who need imminent procedures, are at high risk of bleeding or have significant renal failure. In patients with recurrent VTE despite therapeutic LMWH anticoagulation, the ACCP guidelines recommends increasing the dose of LMWH by 25–30%. While direct oral anticoagulants (DOACs) have advantages including no need for monitoring, none of the major societal guidelines recommend their use in this critical care setting given their lack of timely reversal at some hospitals. Parenteral anticoagulants also have no known drug–drug interactions with COVID-19 therapies, and this may not be true with DOACs.
When are thrombolytics recommended?
Given autopsy findings of patients with COVID-19 revealing significant pulmonary micro- and macro-thrombosis, the question has been raised as to whether there is a role for thrombolytic therapy. There are a number of case series that suggest the use of thrombolytics in patients with severe ARDS and COVID-19 may lead clinical improvement [75,76]. Given this potential benefit, there are a number of ongoing clinical trials investigating the use of parenteral and nebulized thrombolytic therapy for patients with severe COVID-19 ARDS. If a PE is suspected, consultation with a pulmonary embolism response team (PERT) is advised if available. These teams can provide expert advice regarding issues related to diagnosis and management of a PE in patients with COVID-19. If PERT consultation is unavailable, the National PERT Consortium paper has provided an algorithm to assist in decision-making for patients with a suspected PE [52]. Our institution, the Massachusetts General Hospital, has a PERT team which is a multidisciplinary team composed of experts from cardiology, cardiac surgery, emergency medicine, hematology, pulmonary and critical care, radiology, and vascular medicine and delivers immediate and evidence-based care to patients with suspected or confirmed high risk PE [77]. When major societal guidelines do recommend thrombolytic therapy, it is in the clinical context where their use would otherwise be clinically indicated such as STEMI, acute ischemic stroke, or high-risk massive PE with hemodynamic instability and when the benefits outweigh the risks of administration. In general, thrombolytic therapy is not recommended in patients who have a PE and are hemodynamically stable [57].
When to hold anticoagulation?
Most major society’s guidelines (CDC, ISTH-IG, ACF, ASH, SCC-ISTH) advise holding therapeutic and prophylactic anticoagulation in patients who have significant active bleeding and/or severe thrombocytopenia. Both the ACF and SCC-ISTH guidelines suggest holding anticoagulation if platelet count <25 x 10^9/L. ASH recommends holding prophylactic anticoagulation if platelet count is <25 x 10^9/L or fibrinogen <0.5 g/L, and holding therapeutic anticoagulation may necessary if platelet count is <30–50 x 10^9/L or fibrinogen <1.0 g/L. Of note, many patients with COVID-19 may have abnormal baseline PT or PTT, which is not a contraindication to thromboprophylaxis according to the ISTH-IG and ASH guidelines. Therefore, PT and PTT should not be used as a guide to hold prophylactic or therapeutic anticoagulation.
What is the utility of mechanical thromboprophylaxis?
Most of the major society’s guidelines (ACF, ASH, ACCP, SCC-ISTCH, and ACC) recommend or suggest mechanical thromboprophylaxis when pharmacological thromboprophylaxis is contraindicated. Intermittent pneumatic compression devices are the preferred type of mechanical thromboprophylaxis. ACCP suggests against the additional use of mechanical thromboprophylaxis in critically ill patients receiving pharmacological prophylaxis but mentions that its addition is unlikely to cause harm.
What is the appropriate method of monitoring anticoagulation?
Monitoring of patients receiving therapeutic anticoagulation with LMWH
Currently, none of the major society guidelines recommend the routine monitoring of anti-Xa levels of patients receiving LMWH. LMWH is generally preferred if there are no contraindications given the added benefit of not needing routine monitoring. However, the ISTH-IG guidelines state that monitoring of LMWH is advised in patients with severe renal impairment, a patient population generally for which LMWH is not routinely recommended. However, the ACCP guidelines state that body weight adjusted doses for LMWH do not require laboratory monitoring in majority of patients, and the ACF guidelines state that anti-Xa level monitoring is not recommended in patients with elevated PTT levels given the lack of evidence on outcomes for thrombosis or bleeding.
Monitoring of patients receiving therapeutic anticoagulation with UFH
The PTT measures the intrinsic coagulation pathway and is the most commonly used test to monitor UFH [78]. It is not uncommon for patients with COVID-19 to have baseline coagulation abnormalities of PT and PTT. Although these abnormalities are not contraindications to anticoagulation, it may lead to difficulties measuring heparin effectiveness. When in doubt, the majority of society guidelines (ACF, ASH, ACF and ACCP) advise that therapeutic anticoagulation should be monitored with an anti-Xa level rather than PTT. The SCC-ISTH guideline does not make any particular recommendations but does mention that expert clinical guidance statements and clinical pathways from large academic healthcare systems target for therapeutic anticoagulation, an anti-factor Xa level of 0.3–0.7 IU/mL for UFH. While the ACF guideline recommends monitoring of anti-Xa levels to monitor UFH due to potential baseline PTT abnormalities and heparin resistance (>35,000 U heparin over 24 hours), they also mention that it is reasonable to monitor anti-Xa or PTT in patients with normal baseline PTT levels and in those unlikely to have heparin resistance. There is evidence in the value of implementing an anticoagulation protocol using systemic unfractionated heparin, dosed by anti-factor Xa levels. At our institution, The Massachusetts General Hospital, we found that patients with COVID-19 infection on CRRT had lower rates of CRRT filter clotting and loss when using this protocol where systemic unfractionated heparin was dosed by anti-factor Xa levels [74]. However, this needs to be studied further in clinical trials.
Heparin resistance
An important consideration when making decisions about DVT prophylaxis and monitoring of anticoagulation in COVID-19 patients is the concern for heparin resistance, which has been well documented [79]. Heparin resistance should be suspected when disproportionately large doses of heparin are required to achieve therapeutic anticoagulation. This problem is usually due to low heparin concentrations, which results from binding of heparin to acute phase proteins in the context of systemic inflammation. There is also some evidence of AT deficiency in COVID-19 patients which may contribute to suspected heparin resistance [48]. Heparin functions as an anticoagulant by binding to AT, activating it, and then inhibiting clotting factors, most notably factor Xa [80]. A way to measure the capacity of the heparin-AT complex is with anti-Xa levels. It has been well documented that patients with COVID-19 may have artefactual increases in PTT, and therefore measuring anti-Xa levels may be a more accurate way to assess the level of anticoagulation in these situations. Additionally, LMWH can’t be measured with PTT, and as a result, anti-Xa levels may be used to ensure appropriate anticoagulation levels have been achieved if necessary. Unfortunately, not all centers have the capacity to monitor anti-Xa levels.
What is the recommended approach to control active bleeding?
When addressing active bleeding, the major society guidelines recommend holding both prophylactic and therapeutic anticoagulation. However, only the ISTH-IG, ASH and ACC guidelines provide specific recommendations for blood product replacement. The ISTH-IG guidelines recommend transfusing to keep platelet count >50 x 10^9/L, fibrinogen concentrate to target fibrinogen >1.5 g/L, and FFP to target PT ratio <1.5. ASH guidelines recommend transfusing one adult unit of platelets if platelet count <50 x 10^9/L, 4 units of plasma if INR > 1.8 and fibrinogen concentrate (4 g) or cryoprecipitate (10 units) if fibrinogen <1.5 g/L. The ACC guidelines recommend transfusing platelets in patients with active bleeding or requiring invasive procedures if platelet count <20 x 10^9/L, and providing FFP (15 to 25 mL/kg) in patients with active bleeding with either prolonged PT or PTT ratios (>1.5 times normal) as well as transfusing fibrinogen concentrate or cryoprecipitate in patients with persisting severe hypofibrinogenemia (<1.5 g/L).
If volume overload is a concern in patients with active bleeding and severe COVID-19, ASH and ACC guidelines recommend the use of 4 F-PCC (25 u/kg) instead of FFP. The ACC guidelines also state that tranexamic acid should not be routinely used in patients with COVID-19 associated DIC given the lack of existing data. None of the major societies mention the use of TEG to monitor coagulopathy in patients who are actively bleeding.
DIC is an uncommon but serious complication in patients with COVID-19 [81]. It’s important to note that DIC is a clinical diagnosis with exclusion of alternate explanations for coagulation dysfunction. In patients with COVID-19, a superimposed bacterial infection is the most likely precipitant, however other causes such as HIT, drug-induced DIC and malignancy may also be contributing. Treating the underlying cause is the most important component of treating DIC, with transfusion targets the same as for active bleeding. If there is no active bleeding, replacement of fibrinogen and coagulation factors remain controversial. However, if platelet count is <10 x 10^9/L, platelets should be transfused.
Should patients receive post-discharge prophylactic anticoagulation and what regimens are available?
There is no evidence to support post-discharge DVT prophylaxis in patients who were hospitalized with COVID-19 infection. A number of studies have identified very low rates of post-discharge VTE; therefore, there is no universal recommendation for VTE prophylaxis for all patients post-discharge [82–84]. However, in high-risk patients and those who are at low risk of bleeding, the majority of major societal guidelines (CDC, ACF, ASH, ACCP, SCC-ISTH and ACC) state that it is reasonable to consider post-discharge prophylactic anticoagulation. Currently, there is an ongoing randomized trial evaluating the effectiveness and safety of low-dose apixaban in reducing thrombosis in patients who have been discharged from the hospital [85].
At the point of discharge, if a patient is deemed high risk (i.e., D-dimer >2 times the upper limit, reduced mobility, active cancer) of thrombosis and low risk of bleeding, it is reasonable to use criteria from clinical trials involving FDA-approved prophylactic anticoagulation regimens such as LMWH, rivaroxaban and betrixaban for thromboprophylaxis [57]. In terms of how long to provide thromboprophylaxis once discharged, the SCC guidelines recommend following post-discharge prophylactic anticoagulation regimen for 14–30 days. The ACC guideline states that it is reasonable to consider extended prophylaxis with LMWH or DOACs for up to 45 days in patients with high risk for VTE. For those diagnosed with COVID-19, and not admitted to hospital, there is no recommendation for DVT prophylaxis as an outpatient. However, a multicenter randomized controlled trial is underway to evaluate the efficacy and safety of antithrombotic strategies (aspirin compared with low dose and regular dose apixaban, and with placebo) in adults with COVID-19 not requiring hospitalization at time of diagnosis [58]. However, this trial is evaluating outpatients, not patients admitted post-discharge.
Conclusion
CAC is associated with macro- and micro-thrombosis, which can lead to a myriad of different presentations, and may result in multiorgan injury and ultimately death. As a result, important clinical questions regarding the optimal prevention and management of thrombosis has led to many ongoing clinical trials. Whilst data continues to be collected, major hematological societies have put forth recommendations regarding diagnosis, monitoring, and treatment of CAC. Overall, decisions should be made based on the providers understanding of a patient’s medical history, clinical course and perceived risk, in conjunction with the major societal guidelines and results from emerging clinical trial results.
Acknowledgments
We thank the educational division of Roche for allowing us to incorporate this manuscript into their CoagYOUlation platform (http://www.coagYOUlation.com). This manuscript incorporates literature and guidelines available at the time of submission. It is anticipated that the guidelines and practice management provided on the platform will be updated as additional literature becomes available.
Declaration of funding
We would also like to thank Roche for providing support for the article processing fees required in publishing this article and for initial compensation related to the creation of educational content provided in this article.Go to:
Declaration of financial/other relationships
No potential conflict of interest was reported by the author.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Authors contributions
PJL, RPR, EAB, MGC wrote and reviewed the manuscript.
Take home message
COVID-19-associated coagulopathy (CAC) is a well-recognized hematologic complication among patients with severe COVID-19 disease, where macro- and micro-thrombosis can lead to multiorgan injury and failure. In this review, we utilize current societal guidelines to provide a framework for practitioners in managing their patients with CAC.
COVID-19: Coronavirus disease 2019
WHO: World Health Organization
CAC: COVID-19 associated coagulopathy
CDC: Centers for Disease Control and Prevention (CDC),
ISTH-IG: International Society on Thrombosis and Hemostasis interim guidance (ISTH-IG)
ASH: American Society of Hematology (ASH)
ACCP: American College of Chest Physicians
SCC-ISTH: Scientific and Standardization Committee of ISTH
ACF: Anticoagulation Forum
ACC: American College of Cardiology
vWF: von Willebrand Factor
ICU: Intensive Care Unit
TEG: Thromboelastography
ROTEM: Rotational thromboelastometry
DIC: Disseminated intravascular coagulation
PT: Prothrombin time
PTT: Partial thromboplastin time
AT: Antithrombin
MA: Maximum amplitude
INR: International normalized ratio
VTE: Venous thromboembolism
HIT: Heparin-induced thrombocytopenia
SRA: Serotonin release assay
PE: Pulmonary embolism
CTPA: Computed tomography pulmonary angiogram (CTPA)
LMWH: Low molecular weight heparin
UFH: Unfractionated heparin
RCT: Randomized control trial
DOAC: Direct oral anticoagulants
PPE: Personal protective equipment
CRRT: Continuous renal replacement therapyGo to:
Declaration of interest
No potential conflict of interest was reported by the author(s).Go to:
References
1. Organisation WH . WHO coronavirus disease (COVID-19) dashboard. 2021. [Google Scholar]
2. Cui S, Chen S, Li X, et al. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421–1424. [PMC free article] [PubMed] [Google Scholar]
3. Klok FA, Kruip M, NJM VDM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. [PMC free article] [PubMed] [Google Scholar]
4. Lax SF, Skok K, Zechner P, et al. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series. Ann Intern Med. 2020;173(5):350–361. [PMC free article] [PubMed] [Google Scholar]
5. Nopp S, Moik F, Jilma B, et al. Risk of venous thromboembolism in patients with COVID-19: a systematic review and meta-analysis. Res Pract Thromb Haemost. 2020;4(7):1178–1191. [PMC free article] [PubMed] [Google Scholar]
6. Siddiqi HK, Mehra MRCOVID-19. illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39(5):405–407. [PMC free article] [PubMed] [Google Scholar]
7. Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–128. [PMC free article] [PubMed] [Google Scholar]
8. White-Dzuro G, Gibson LE, Zazzeron L, et al. Multisystem effects of COVID-19: a concise review for practitioners. Postgrad Med. 2021;133(1):20–27. [PMC free article] [PubMed] [Google Scholar]
9. Moores LK, Tritschler T, Brosnahan S, et al. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019 CHEST guideline and expert panel report. Chest. 2020;158(3):1143–1163. [PMC free article] [PubMed] [Google Scholar]
10. Barnes GD, Burnett A, Allen A, et al. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis. 2020;50(1):72–81. [PMC free article] [PubMed] [Google Scholar]
11. Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-Art review. J Am Coll Cardiol. 2020;75(23):2950–2973. [PMC free article] [PubMed] [Google Scholar]
12. Spyropoulos AC, Levy JH, Ageno W, et al. Scientific and Standardization Committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18(8):1859–1865. [PMC free article] [PubMed] [Google Scholar]
13. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023–1026. [PubMed] [Google Scholar]
14. Bilaloglu S, Aphinyanaphongs Y, Jones S, et al. Thrombosis in hospitalized patients with COVID-19 in a New York city health system. JAMA. 2020;324(8):799–801. [PMC free article] [PubMed] [Google Scholar]
15. Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. [PMC free article] [PubMed] [Google Scholar]
16. Giustozzi M, Vedovati MC, Agnelli G.. Venous thromboembolism and COVID-19: mind the gap between clinical epidemiology and patient management. Eur J Intern Med. 2020;82:18–20. [PMC free article] [PubMed] [Google Scholar]
17. Moll M, Zon RL, Sylvester KW, et al. VTE in ICU patients with COVID-19. Chest. 2020;158(5):2130–2135. [PMC free article] [PubMed] [Google Scholar]
18. Jimenez D, Garcia-Sanchez A, Rali P, et al. Incidence of VTE and bleeding among hospitalized patients with coronavirus disease 2019: a systematic review and meta-analysis. Chest. 2020. [PMC free article] [PubMed] [Google Scholar]
19. Poissy J, Goutay J, Caplan M, et al. Pulmonary embolism in patients with COVID-19: awareness of an increased prevalence. Circulation. 2020;142(2):184–186. [PubMed] [Google Scholar]
20. Al-Samkari H, Karp Leaf RS, Dzik WH, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136(4):489–500. [PMC free article] [PubMed] [Google Scholar]
21. Boyd S, Martin-Loeches I. The incidence of venous thromboembolism in critically ill patients with COVID-19 compared with critically ill non-COVID patients. Ir J Med Sci. 2021. DOI:10.1007/s11845-020-02503-0 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
22. Klok FA, Kruip M, NJM VDM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. [PMC free article] [PubMed] [Google Scholar]
23. Menter T, Haslbauer JD, Nienhold R, et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77(2):198–209. [PMC free article] [PubMed] [Google Scholar]
24. Lowenstein CJ, Solomon SD, Severe COVID-19. Is a microvascular disease. Circulation. 2020;142(17):1609–1611. [PMC free article] [PubMed] [Google Scholar]
25. Oxley TJ, Mocco J, Majidi S, et al. Large-Vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020;382(20):e60. [PMC free article] [PubMed] [Google Scholar]
26. Etkin Y, Conway AM, Silpe J, et al. Acute arterial thromboembolism in patients with COVID-19 in the New York city area. Ann Vasc Surg. 2021;70:290–294. [PMC free article] [PubMed] [Google Scholar]
27. Bellosta R, Luzzani L, Natalini G, et al. Acute limb ischemia in patients with COVID-19 pneumonia. J Vasc Surg. 2020;72(6):1864–1872. [PMC free article] [PubMed] [Google Scholar]
28. Lillicrap D. Disseminated intravascular coagulation in patients with 2019-nCoV pneumonia. J Thromb Haemost. 2020;18(4):786–787. [PMC free article] [PubMed] [Google Scholar]
29. Iba T, Levy JH, Levi M, et al. Coagulopathy in COVID-19. J Thromb Haemost. 2020;18(9):2103–2109. [PMC free article] [PubMed] [Google Scholar]
30. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. [PMC free article] [PubMed] [Google Scholar]
31. Iba T, Levy JH. Inflammation and thrombosis: roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis. J Thromb Haemost. 2018;16(2):231–241. [PubMed] [Google Scholar]
32. Grobler C, Maphumulo SC, Grobbelaar LM, et al. Covid-19: the rollercoaster of fibrin(Ogen), D-Dimer, Von Willebrand Factor, P-Selectin and their interactions with endothelial cells, platelets and erythrocytes. Int J Mol Sci. 2020;21(14):5168. [PMC free article] [PubMed] [Google Scholar]
33. Panigada M, Bottino N, Tagliabue P, et al. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18(7):1738–1742. [PubMed] [Google Scholar]
34. Goshua G, Pine AB, Meizlish ML, et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7(8):e575–e82. [PMC free article] [PubMed] [Google Scholar]
35. Mortus JR, Manek SE, Brubaker LS, et al. Thromboelastographic results and hypercoagulability syndrome in patients with coronavirus disease 2019 who are critically Ill. JAMA Network Open. 2020;3(6):e2011192. [PMC free article] [PubMed] [Google Scholar]
36. Driggin E, Madhavan MV, Bikdeli B, et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol. 2020;75(18):2352–2371. [PMC free article] [PubMed] [Google Scholar]
37. Adam SS, Key NS, Greenberg CS. D-dimer antigen: current concepts and future prospects. Blood. 2009;113(13):2878–2887. [PubMed] [Google Scholar]
38. Li J, Liu Z, Wu G, et al. d-dimer as a prognostic indicator in critically Ill patients hospitalized with COVID-19 in Leishenshan hospital, Wuhan, China. Front Pharmacol. 2020;11:600592. [PMC free article] [PubMed] [Google Scholar]
39. Battinelli EMCOVID-19. concerns aggregate around platelets. Blood. 2020;136(11):1221–1223. [PMC free article] [PubMed] [Google Scholar]
40. Rampotas A, Pavord S. Platelet aggregates, a marker of severe COVID-19 disease. J Clin Pathol. 2020. DOI:10.1136/jclinpath-2020-206933 [PubMed] [CrossRef] [Google Scholar]
41. Manne BK, Denorme F, Middleton EA, et al. Platelet gene expression and function in patients with COVID-19. Blood. 2020;136(11):1317–1329. [PMC free article] [PubMed] [Google Scholar]
42. Yang X, Yang Q, Wang Y, et al. Thrombocytopenia and its association with mortality in patients with COVID-19. J Thromb Haemost. 2020;18(6):1469–1472. [PubMed] [Google Scholar]
43. Bowles L, Platton S, Yartey N, et al. Lupus anticoagulant and abnormal coagulation tests in patients with Covid-19. N Engl J Med. 2020;383(3):288–290. [PMC free article] [PubMed] [Google Scholar]
44. Harzallah I, Debliquis A, Drenou B. Lupus anticoagulant is frequent in patients with Covid-19. J Thromb Haemost. 2020;18(8):2064–2065. [PMC free article] [PubMed] [Google Scholar]
45. Giannakopoulos B, Passam F, Ioannou Y, et al. How we diagnose the antiphospholipid syndrome. Blood. 2009;113(5):985–994. [PubMed] [Google Scholar]
46. Devreese KMJ, Linskens EA, Benoit D, et al. Antiphospholipid antibodies in patients with COVID-19: a relevant observation? J Thromb Haemost. 2020;18(9):2191–2201. [PMC free article] [PubMed] [Google Scholar]
47. Reyes Gil M, Barouqa M, Szymanski J, et al. Assessment of Lupus anticoagulant positivity in patients with coronavirus disease 2019 (COVID-19). JAMA Network Open. 2020;3(8):e2017539. [PubMed] [Google Scholar]
48. Mills K, Sobukonla T, Lee M, et al. DECODING COVID-19: a NOVEL ROLE OF ANTITHROMBIN DEFICIENCY IN THE NOVEL CORONAVIRUS. Chest. 2020;158(4):4. [Google Scholar]
49. Connors JM, Levy JH. Thrombo inflammation and the hypercoagulability of COVID-19. J Thromb Haemost. 2020;18(7):1559–1561. [PubMed] [Google Scholar]
50. Zhang B, Zhou X, Qiu Y, et al. Clinical characteristics of 82 cases of death from COVID-19. PLoS One. 2020;15(7):e0235458. [PMC free article] [PubMed] [Google Scholar]
51. Asakura H, Ogawa H. COVID-19-associated coagulopathy and disseminated intravascular coagulation. Int J Hematol. 2021;113(1):45–57. [PMC free article] [PubMed] [Google Scholar]
52. Rosovsky RP, Grodzin C, Channick R, et al. Diagnosis and treatment of pulmonary embolism during the coronavirus disease 2019 pandemic: a position paper from the national PERT consortium. Chest. 2020;158(6):2590–2601. [PMC free article] [PubMed] [Google Scholar]
53. Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. [PMC free article] [PubMed] [Google Scholar]
54. Zhang L, Yan X, Fan Q, et al. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 2020;18(6):1324–1329. [PMC free article] [PubMed] [Google Scholar]
55. Linkins LA, Takach Lapner S. Review of D-dimer testing: good, bad, and ugly. Int J Lab Hematol. 2017;39(Suppl 1):98–103. [PubMed] [Google Scholar]
56. Yao Y, Cao J, Wang Q, et al. D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: a case control study. J Intensive Care. 2020;8(1):49. [PMC free article] [PubMed] [Google Scholar]
57. Flaczyk A, Rosovsky RP, Reed CT, et al. Comparison of published guidelines for management of coagulopathy and thrombosis in critically ill patients with COVID 19: implications for clinical practice and future investigations. Crit Care. 2020;24(1):559. [PMC free article] [PubMed] [Google Scholar]
58. Connors JM. 2021. ACTIV-IV COVID-19 outpatient thrombosis prevention trial.
59. Young E. The anti-inflammatory effects of heparin and related compounds. Thromb Res. 2008;122(6):743–752. [PubMed] [Google Scholar]
60. Hippensteel JA, LaRiviere WB, Colbert JF, et al. Heparin as a therapy for COVID-19: current evidence and future possibilities. Am J Physiol Lung Cell Mol Physiol. 2020;319(2):L211–L7. [PMC free article] [PubMed] [Google Scholar]
61. Spiezia L, Boscolo A, Poletto F, et al. COVID-19-Related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. 2020;120(6):998–1000. [PMC free article] [PubMed] [Google Scholar]
62. Lin L, Lu L, Cao W, et al. Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect. 2020;9(1):727–732. [PMC free article] [PubMed] [Google Scholar]
63. Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. [PubMed] [Google Scholar]
64. Health NIo . Full-dose blood thinners decreased need for life support and improved outcome in hospitalized COVID-19 patients. 2021. [Google Scholar]
65. Cuker A, Tseng EK, Nieuwlaat R, et al. American Society of Hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19. Blood Adv. 2021;5(3):872–888. [PMC free article] [PubMed] [Google Scholar]
66. Nadkarni GN, Lala A, Bagiella E, et al. Anticoagulation, bleeding, mortality, and pathology in hospitalized patients with coVID-19. J Am Coll Cardiol. 2020;76(16):1815–1826. [PMC free article] [PubMed] [Google Scholar]
67. Wang L, Li H, Gu X, et al. Effect of Antiplatelet therapy on acute respiratory distress syndrome and mortality in critically Ill patients: a meta-Analysis. PLoS One. 2016;11(5):e0154754. [PMC free article] [PubMed] [Google Scholar]
68. D’Souza R, Malhame I, Teshler L, et al. A critical review of the pathophysiology of thrombotic complications and clinical practice recommendations for thromboprophylaxis in pregnant patients with COVID-19. Acta Obstet Gynecol Scand. 2020;99(9):1110–1120. [PMC free article] [PubMed] [Google Scholar]
69. DiNicolantonio JJ, Barroso-Aranda J. Harnessing adenosine A2A receptors as a strategy for suppressing the lung inflammation and thrombotic complications of COVID-19: potential of pentoxifylline and dipyridamole. Med Hypotheses. 2020;143:110051. [PMC free article] [PubMed] [Google Scholar]
70. Chow JH, Khanna AK, Kethireddy S, et al. Aspirin use is associated with decreased mechanical ventilation, ICU admission, and in-hospital mortality in hospitalized patients with COVID-19. Anesth Analg. 2020. [PubMed] [Google Scholar]
71. Salah HM, Mehta JL. Meta-Analysis of the effect of aspirin on mortality in COVID-19. Am J Cardiol. 2021. [PMC free article] [PubMed] [Google Scholar]
72. Trial R. Aspirin to be investigated as a possible treatment for COVID-19 in the RECOVERY trial.
73. Maurer LR, Luckhurst CM, Hamidi A, et al. A low dose heparinized saline protocol is associated with improved duration of arterial line patency in critically ill COVID-19 patients. J Crit Care. 2020;60:253–259. [PMC free article] [PubMed] [Google Scholar]
74. Endres P, Rosovsky R, Zhao S, et al. Filter clotting with continuous renal replacement therapy in COVID-19. J Thromb Thrombolysis. 2020. [PMC free article] [PubMed] [Google Scholar]
75. Barrett CD, Oren-Grinberg A, Chao E, et al. Rescue therapy for severe COVID-19-associated acute respiratory distress syndrome with tissue plasminogen activator: a case series. J Trauma Acute Care Surg. 2020;89(3):453–457. [PMC free article] [PubMed] [Google Scholar]
76. Wang J, Hajizadeh N, Moore EE, et al. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J Thromb Haemost. 2020;18(7):1752–1755. [PMC free article] [PubMed] [Google Scholar]
77. Kabrhel C, Rosovsky R, Channick R, et al. A multidisciplinary pulmonary embolism response team: initial 30-Month experience with a novel approach to delivery of care to patients with submassive and massive pulmonary embolism. Chest. 2016;150(2):384–393. [PubMed] [Google Scholar]
78. Streng AS, Delnoij TSR, Mulder MMG, et al. Monitoring of unfractionated Heparin in severe COVID-19: an observational study of patients on CRRT and ECMO. TH Open. 2020;4(4):e365–e75. [PMC free article] [PubMed] [Google Scholar]
79. White D, MacDonald S, Bull T, et al. Heparin resistance in COVID-19 patients in the intensive care unit. J Thromb Thrombolysis. 2020;50(2):287–291. [PMC free article] [PubMed] [Google Scholar]
80. Trunfio M, Salvador E, Cabodi D, et al. Anti-Xa monitoring improves low-molecular-weight heparin effectiveness in patients with SARS-CoV-2 infection. Thromb Res. 2020;196:432–434. [PMC free article] [PubMed] [Google Scholar]
81. Zhou X, Cheng Z, Luo L, et al. Incidence and impact of disseminated intravascular coagulation in COVID-19 a systematic review and meta-analysis. Thromb Res. 2021;201:23–29. [PMC free article] [PubMed] [Google Scholar]
82. Patell R, Bogue T, Koshy A, et al. Postdischarge thrombosis and hemorrhage in patients with COVID-19. Blood. 2020;136(11):1342–1346. [PMC free article] [PubMed] [Google Scholar]
83. Roberts LN, Whyte MB, Georgiou L, et al. Postdischarge venous thromboembolism following hospital admission with COVID-19. Blood. 2020;136(11):1347–1350. [PMC free article] [PubMed] [Google Scholar]
84. Hill JB, Garcia D, Crowther M, et al. Frequency of venous thromboembolism in 6513 patients with COVID-19: a retrospective study. Blood Adv. 2020;4(21):5373–5377. [PMC free article] [PubMed] [Google Scholar]
85. Ortel T. 2021. COVID-19 thrombosis prevention trials: post-hospital thromboprophylaxis.
Formats:
Share
Similar articles in PubMed
- Approach to the Patient with COVID-19-Associated Thrombosis: A Case-Based Review.[Oncologist. 2020]
- Anticoagulation for COVID-19 Patients: A Bird’s-Eye View.[Clin Appl Thromb Hemost. 2021]
- Comparison of published guidelines for management of coagulopathy and thrombosis in critically ill patients with COVID 19: implications for clinical practice and future investigations.[Crit Care. 2020]
- COVID-19 and thrombosis: From bench to bedside.[Trends Cardiovasc Med. 2021]
- Covid-19-induced coagulopathy and observed benefits with anticoagulation.[Transfus Apher Sci. 2020]
Links
Recent Activity
- Nuts and bolts of COVID-19 associated coagulopathy: the essentials for managemen…Nuts and bolts of COVID-19 associated coagulopathy: the essentials for management and treatmentTaylor & Francis Public Health Emergency Collection. 2021; ()1
- Genetics Insight for COVID-19 Susceptibility and Severity: A ReviewGenetics Insight for COVID-19 Susceptibility and Severity: A ReviewFrontiers in Immunology. 2021; 12()
- Ground-glass opacity (GGO): a review of the differential diagnosis in the era of…Ground-glass opacity (GGO): a review of the differential diagnosis in the era of COVID-19Nature Public Health Emergency Collection. 2021; 39(8)721
- COVID-19 targets human adrenal glandsCOVID-19 targets human adrenal glandsElsevier Public Health Emergency Collection. 2022 Jan; 10(1)13
- Potential pharmacologic treatments for COVID-19 smell and taste loss: A comprehe…Potential pharmacologic treatments for COVID-19 smell and taste loss: A comprehensive reviewElsevier Public Health Emergency Collection. 2021 Dec 5; 912()174582
- COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal.[J Heart Lung Transplant. 2020]
- Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19.[N Engl J Med. 2020]
- Prevention, Diagnosis, and Treatment of VTE in Patients With Coronavirus Disease 2019: CHEST Guideline and Expert Panel Report.[Chest. 2020]
- Thrombosis in Hospitalized Patients With COVID-19 in a New York City Health System.[JAMA. 2020]
- High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study.[Intensive Care Med. 2020]
- Venous thromboembolism and COVID-19: Mind the gap between clinical epidemiology and patient management.[Eur J Intern Med. 2020]
- VTE in ICU Patients With COVID-19.[Chest. 2020]
- Risk of venous thromboembolism in patients with COVID-19: A systematic review and meta-analysis.[Res Pract Thromb Haemost. 2020]
- Incidence of VTE and Bleeding Among Hospitalized Patients With Coronavirus Disease 2019: A Systematic Review and Meta-analysis.[Chest. 2021]
- Pulmonary Embolism in Patients With COVID-19: Awareness of an Increased Prevalence.[Circulation. 2020]
- COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection.[Blood. 2020]
- Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young.[N Engl J Med. 2020]
- Acute Arterial Thromboembolism in Patients with COVID-19 in the New York City Area.[Ann Vasc Surg. 2021]
- Acute limb ischemia in patients with COVID-19 pneumonia.[J Vasc Surg. 2020]
- Review Multisystem effects of COVID-19: a concise review for practitioners.[Postgrad Med. 2021]
- Disseminated intravascular coagulation in patients with 2019-nCoV pneumonia.[J Thromb Haemost. 2020]
- Review Coagulopathy in COVID-19.[J Thromb Haemost. 2020]
- Endothelial cell infection and endotheliitis in COVID-19.[Lancet. 2020]
- Review Inflammation and thrombosis: roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis.[J Thromb Haemost. 2018]
- Severe COVID-19 Is a Microvascular Disease.[Circulation. 2020]
- Review Covid-19: The Rollercoaster of Fibrin(Ogen), D-Dimer, Von Willebrand Factor, P-Selectin and Their Interactions with Endothelial Cells, Platelets and Erythrocytes.[Int J Mol Sci. 2020]
- Hypercoagulability of COVID-19 patients in intensive care unit: A report of thromboelastography findings and other parameters of hemostasis.[J Thromb Haemost. 2020]
- Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study.[Lancet Haematol. 2020]
- Thromboelastographic Results and Hypercoagulability Syndrome in Patients With Coronavirus Disease 2019 Who Are Critically Ill.[JAMA Netw Open. 2020]
- Review Cardiovascular Considerations for Patients, Health Care Workers, and Health Systems During the COVID-19 Pandemic.[J Am Coll Cardiol. 2020]
- Review D-dimer antigen: current concepts and future prospects.[Blood. 2009]
- COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection.[Blood. 2020]
- D-Dimer as a Prognostic Indicator in Critically Ill Patients Hospitalized With COVID-19 in Leishenshan Hospital, Wuhan, China.[Front Pharmacol. 2020]
- COVID-19 concerns aggregate around platelets.[Blood. 2020]
- Platelet aggregates, a marker of severe COVID-19 disease.[J Clin Pathol. 2021]
- Platelet gene expression and function in patients with COVID-19.[Blood. 2020]
- COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection.[Blood. 2020]
- Thrombocytopenia and its association with mortality in patients with COVID-19.[J Thromb Haemost. 2020]
- Lupus Anticoagulant and Abnormal Coagulation Tests in Patients with Covid-19.[N Engl J Med. 2020]
- Lupus anticoagulant is frequent in patients with Covid-19.[J Thromb Haemost. 2020]
- Review How we diagnose the antiphospholipid syndrome.[Blood. 2009]
- High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study.[Intensive Care Med. 2020]
- Antiphospholipid antibodies in patients with COVID-19: A relevant observation?[J Thromb Haemost. 2020]
- Assessment of Lupus Anticoagulant Positivity in Patients With Coronavirus Disease 2019 (COVID-19).[JAMA Netw Open. 2020]
- VTE in ICU Patients With COVID-19.[Chest. 2020]
- Incidence of thrombotic complications in critically ill ICU patients with COVID-19.[Thromb Res. 2020]
- Thrombosis in Hospitalized Patients With COVID-19 in a New York City Health System.[JAMA. 2020]
- Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction.[Histopathology. 2020]
- Thromboinflammation and the hypercoagulability of COVID-19.[J Thromb Haemost. 2020]
- COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection.[Blood. 2020]
- Clinical characteristics of 82 cases of death from COVID-19.[PLoS One. 2020]
- Review COVID-19-associated coagulopathy and disseminated intravascular coagulation.[Int J Hematol. 2021]
- Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia.[J Thromb Haemost. 2020]
- ISTH interim guidance on recognition and management of coagulopathy in COVID-19.[J Thromb Haemost. 2020]
- D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19.[J Thromb Haemost. 2020]
- Review Review of D-dimer testing: Good, Bad, and Ugly.[Int J Lab Hematol. 2017]
- D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: a case control study.[J Intensive Care. 2020]
- Review Comparison of published guidelines for management of coagulopathy and thrombosis in critically ill patients with COVID 19: implications for clinical practice and future investigations.[Crit Care. 2020]
- Prevention, Diagnosis, and Treatment of VTE in Patients With Coronavirus Disease 2019: CHEST Guideline and Expert Panel Report.[Chest. 2020]
- Review Cardiovascular Considerations for Patients, Health Care Workers, and Health Systems During the COVID-19 Pandemic.[J Am Coll Cardiol. 2020]
- Review The anti-inflammatory effects of heparin and related compounds.[Thromb Res. 2008]
- Review The anti-inflammatory effects of heparin and related compounds.[Thromb Res. 2008]
- Review Heparin as a therapy for COVID-19: current evidence and future possibilities.[Am J Physiol Lung Cell Mol Physiol. 2020]
- VTE in ICU Patients With COVID-19.[Chest. 2020]
- Incidence of thrombotic complications in critically ill ICU patients with COVID-19.[Thromb Res. 2020]
- COVID-19-Related Severe Hypercoagulability in Patients Admitted to Intensive Care Unit for Acute Respiratory Failure.[Thromb Haemost. 2020]
- Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum.[J Thromb Thrombolysis. 2020]
- Scientific and Standardization Committee communication: Clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19.[J Thromb Haemost. 2020]
- Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia.[Emerg Microbes Infect. 2020]
- Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy.[J Thromb Haemost. 2020]
- Review The anti-inflammatory effects of heparin and related compounds.[Thromb Res. 2008]
- American Society of Hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19.[Blood Adv. 2021]
- Anticoagulation, Bleeding, Mortality, and Pathology in Hospitalized Patients With COVID-19.[J Am Coll Cardiol. 2020]
- Effect of Antiplatelet Therapy on Acute Respiratory Distress Syndrome and Mortality in Critically Ill Patients: A Meta-Analysis.[PLoS One. 2016]
- Meta-Analysis of the Effect of Aspirin on Mortality in COVID-19.[Am J Cardiol. 2021]
- A low dose heparinized saline protocol is associated with improved duration of arterial line patency in critically ill COVID-19 patients.[J Crit Care. 2020]
- Filter clotting with continuous renal replacement therapy in COVID-19.[J Thromb Thrombolysis. 2021]
- Rescue therapy for severe COVID-19-associated acute respiratory distress syndrome with tissue plasminogen activator: A case series.[J Trauma Acute Care Surg. 2020]
- Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): A case series.[J Thromb Haemost. 2020]
- Review Diagnosis and Treatment of Pulmonary Embolism During the Coronavirus Disease 2019 Pandemic: A Position Paper From the National PERT Consortium.[Chest. 2020]
- A Multidisciplinary Pulmonary Embolism Response Team: Initial 30-Month Experience With a Novel Approach to Delivery of Care to Patients With Submassive and Massive Pulmonary Embolism.[Chest. 2016]
- Review Comparison of published guidelines for management of coagulopathy and thrombosis in critically ill patients with COVID 19: implications for clinical practice and future investigations.[Crit Care. 2020]
- Monitoring of Unfractionated Heparin in Severe COVID-19: An Observational Study of Patients on CRRT and ECMO.[TH Open. 2020]
- Filter clotting with continuous renal replacement therapy in COVID-19.[J Thromb Thrombolysis. 2021]
- Heparin resistance in COVID-19 patients in the intensive care unit.[J Thromb Thrombolysis. 2020]
- Anti-Xa monitoring improves low-molecular-weight heparin effectiveness in patients with SARS-CoV-2 infection.[Thromb Res. 2020]