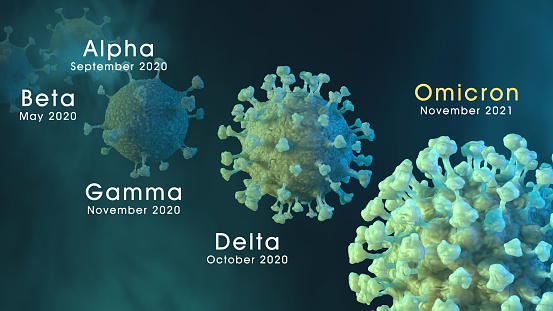
Non-pharmaceutical interventions, vaccination, and the SARS-CoV-2 delta variant in England: a mathematical modelling study
Lancet. 2021 Nov 13; 398(10313): 1825–1835. doi: 10.1016/S0140-6736(21)02276-5 PMCID: PMC855091 Authors: Raphael Sonabend, PhD,a,† Lilith K Whittles, PhD,a,b,c,† Natsuko Imai, PhD,a,† Pablo N Perez-Guzman, MD,a,† Edward S Knock, PhD,a,b,† Thomas Rawson, DPhil,a Katy A M Gaythorpe, PhD,a Bimandra A Djaafara,…[...]
Read More