Authors: Philip C Robinson, Duncan Richards, Helen L Tanner, Marc Feldmann
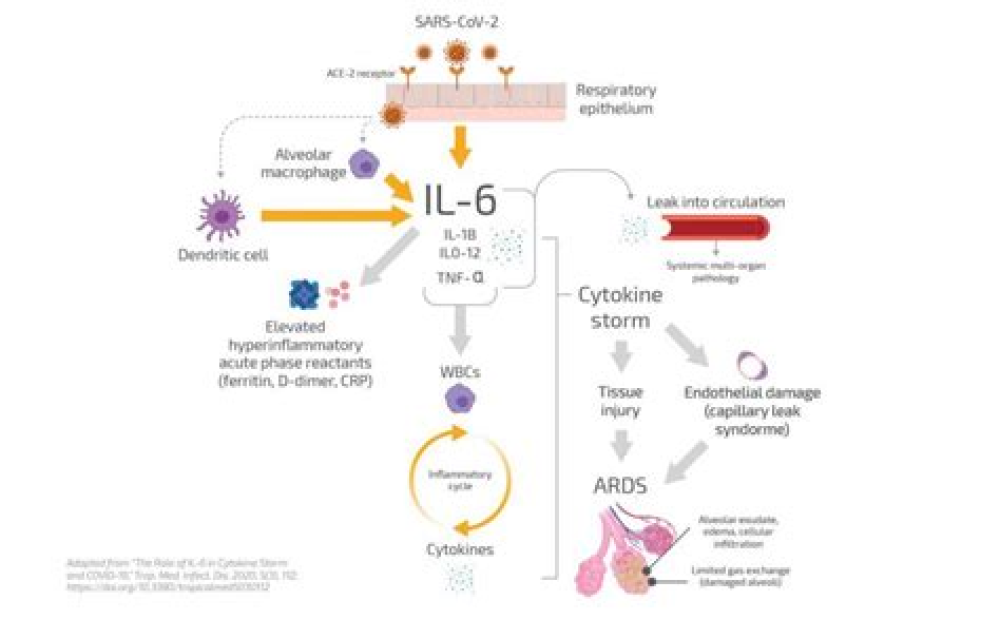
The COVID-19 pandemic continues to wreak havoc on global health-care systems and to claim an increasing number of lives. Although some treatments have shown promise, including dexamethasone and remdesivir, problems remain with access to medication and high mortality despite treatment. Patient selection also appears to be critical, with some patient groups benefitting from treatment, but not others. One potential treatment that deserves higher priority in COVID-19 trials, based on the documented evidence of its effects, is the biological agent anti-TNF.Feldmann and colleagues1 described the rationale for trialling anti-TNF therapies in COVID-19. These therapies neutralise TNF, a major component of the cytokine response that is part of the damaging excess inflammatory phase of COVID-19, which is termed hyperinflammation or cytokine release syndrome. This hyperinflammatory response in COVID-19 is characterised by elevated concentrations of serum TNF, interleukin (IL)-6, and IL-8, but relatively little IL-1.2 However, IL-1 has a short serum half-life, and mononuclear transcriptome data show that genes and pathways upregulated by TNF, IL-1β, and type I interferon predominate.3 A major component of deteriorating lung function in patients with COVID-19 is capillary leak, a result of inflammation driven by key inflammatory cytokines: TNF, IL-1, IL-6, and vascular endothelial growth factor. Administration of anti-TNF to patients for treatment of autoimmune disease leads to reductions in all of these key inflammatory cytokines.4, 5 It is therefore conceivable that anti-TNF therapy could reduce inflammation-driven capillary leak in COVID-19 and have a major impact on the need for ventilation and mortality.
For More Information: https://www.thelancet.com/journals/lanrhe/article/PIIS2665-9913(20)30309-X/fulltext