Yu-Jin Choi, Jin-Seok Lee, Jin-Yong Joung, BMC Infectious Diseases volume 25, Article number: 611 (2025) Cite this article
Abstract
Purpose
The COVID-19 pandemic has led to the emergence of a secondary public health crisis known as Long COVID. It is estimated that approximately 10% of individuals who contact COVID-19 develop Long COVID, with fatigue and brain fog being among the most commonly reported and debilitating symptoms. However, no standardized or effective treatments are currently available. This observational study aimed to evaluate the efficacy of MYPplus, an herbal formulation composed of Astragali Radix, Salviae Radix, and Aquilariae Lignum, in alleviating fatigue and brain fog in patients with Long COVID.
Methods
Subjects with a score of 60 or higher on the Modified Korean version of the Chalder Fatigue scale (mKCFQ11) or a brain fog rating of 5 or higher on the visual analogue scale (VAS) took two capsules of MYPplus (500 mg per capsule) twice daily for 4 weeks. Changes in symptoms were assessed using the mKCFQ11, Multidimensional Fatigue Inventory (MFI-20), Fatigue VAS, Brain fog VAS, and overall quality of life using the Short-Form Health Survey (SF-12). Additionally, levels of three cytokines (TNF-α, TGF-β, IFN- γ) and cortisol were measured.
Results
Fifty participants successfully completed the 4-week administration with MYPplus. At baseline, fatigue severity was 75.3 ± 10.9 in mKCFQ11, 70.9 ± 11.2 in MFI-20, 7.5 ± 1.2 in Fatigue VAS, 8.4 ± 1.1 in Brain fog VAS, and 45.3 ± 17.8 in SF-12. All parameters significantly improved (p < 0.01), with a decrease of 46% in mKCFQ11, 26% in MFI-20, 49% in Fatigue VAS, and 52% in Brain fog VAS, and an increase of 59% in SF-12, respectively. Unlikely others, the plasma level of TGF-β showed a declining pattern after MYPplus administration (from 765.0 ± 1759.7 to 243.9 ± 708.1 pg/mL, p = 0.07). No safety concerns were observed.
Conclusion
This pilot observational study suggests the clinical potential of MYPplus for managing patients with Long COVID, focusing on fatigue-related symptoms and quality of life. Further studies are required to confirm its efficacy and safety using large-scale randomized placebo-controlled trials in the future.
Protocol registration
This study has been retrospectively registered with the identifier number KCT0008948 on https://cris.nih.go.kr, as of 27/10/23.
Background
After recovering from acute COVID-19, approximately 43% of patients worldwide–around 200 million people–experience long-term health consequences [1]. Known as Long COVID, these sequelae involve persistent or new symptoms that emerge 3 months after infection and last for at least 2 months without an alternative diagnosis [2].
Among the various symptoms, fatigue is particularly persistent, sometimes lasting for years after COVID-19 infection. A 2-year follow-up study on Long COVID reported a 34.8% prevalence of fatigue, along with cognitive impairments such as amnesia (30.3%) and concentration difficulties (24.2%) [3]. Fatigue, post-exertional malaise (PEM), and cognitive dysfunction have led to reduced working hours in 45.2% of cases and job loss in 22.3% [4], contributing to an estimated annual economic burden of $1 trillion–approximately 1% of the global economy [5]. Recognizing its impact, the Americans with Disabilities Act (ADA) classified Long COVID as a disability in 2021, citing brain fog as a major factor limiting daily activities [6].
Despite the growing recognition of Long COVID, its underlying pathophysiology remains poorly understood. Proposed mechanisms include immune dysregulation, autoimmunity, and dysfunctional neurological signaling, all of which contribute to chronic inflammation and oxidative stress [7]. Consequently, several treatment approaches have been explored, including anti-inflammatory drugs, antibiotics, and antioxidants [8,9,10]. While some clinical trials have shown symptomatic improvement, their limited internal and external validity has restricted the generalizability of findings [11]. As a result, there remains no approved treatment for Long COVID.
Notably, Long COVID shares many clinical and pathophysiological similarities with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) [12], a debilitating condition characterized by unexplained chronic fatigue and exhaustion, often linked to viral infections–commonly referred to as post-viral fatigue syndrome (PVFS) [13]. Both conditions are associated with increased pro-inflammatory cytokine levels and hypothalamic-pituitary-adrenal (HPA) axis dysregulation [14, 15]. Given these similarities, therapies that have demonstrated efficacy in ME/CFS may hold potential for Long COVID. One such treatment is Myelophil (MYP), a formulation of Astragali radix and Salviae radix, which has been used clinically in South Korea for ME/CFS [16]. A recent phase 2 randomized controlled trial (RCT) demonstrated its efficacy, particularly in patients with severe fatigue [17]. Additionally, Aquilariae Lignum, a traditional herbal medicine used for inflammation and mental disorders [18], has shown pharmacological effects against neuroinflammation and hippocampal excitotoxicity [19, 20]. Since 2020, MYP combined with Aquilariae Lignum (MYPplus) has been prescribed at the author’s hospital for patients with ME/CFS and brain fog. Preliminary clinical observations suggest potential benefits, yet no systemic data exist regarding its efficacy in Long COVID.
Given the absence of approved treatments for Long COVID, this observational study evaluates the efficacy and safety of MYPplus in alleviating fatigue and brain fog under routine clinical conditions. By assessing symptom severity, quality of life, and biomarkers related to inflammation and HPA axis function, this study aims to provide preliminary evidence for MYPplus as a potential therapeutic option.
Methods
Study oversight
A pilot observational study was conducted at Daejeon Korean Medicine Hospital of Daejeon University. This trial evaluated the efficacy and safety of a three-herbal extract, MYPplus, on Long COVID-related fatigue and/or brain fog symptoms and impaired quality of life. The study protocol was designed based on the principles outlined in the Declaration of Helsinki and received ethical clearance from the Institutional Review Board (IRB) under the reference number DJDSKH-21-BM-19. This study is registered at https://cris.nih.go.kr with the identifier number KCT0008948. All patients provided written informed consent.
Study design and participants
As a pilot observational study, we enrolled 50 patients between May 2022 and February 2023 according to the inclusion criteria as follows: (1) aged over 12 years and under 71 years; (2) experiencing severe fatigue and/or brain fog even after complete recovery from COVID-19; and (3) having a score of 60 or higher on the Modified Korean version of the Chalder Fatigue Scale (mKCFQ11) or a brain fog rating of 5 or higher on the Visual Analog Scale (VAS). The exclusion criteria were as follows: (1) individuals with potential alternative causes of fatigue, such as cancer, hepatic disease, cardiovascular disease, kidney or thyroid dysfunction, or severe anemia; (2) those taking medications for fatigue or brain fog; or (3) participants who had recently participated in other clinical trials within the past 6 months. Full inclusion and exclusion criteria are provided in Supplementary Table 1.
Sample size estimation
To determine the required sample size, G*Power software, version 3.1.9.7, was applied in this study [21]. According to Cohen’s law, with a significance level of 0.05, an effect size of 0.5, and a power of 0.95 set for the paired t-test, the minimum required sample size was found to be 45 subjects. Assuming that 10 ∼ 20% of participants would drop out or be lost to follow-up, 50 participants were determined.
Intervention and choice of MYPplus
Fifty participants were instructed to orally consume a total of 2,000 mg of MYPplus per day (two capsules within 10 min to 1 h after meals, twice daily) for a 4-week period. Manufactured by Hankook BioPharm Pharmacy, the MYPplus capsule abided by the Korean Good Manufacturing Practice guidelines. Each capsule contained 450 mg of a 30% ethanol extract (Astragali Radix and Salviae Radix) and 50 mg of Aquilariae Lignum grinding powder. Molecular fingerprinting of MYP and Aquilariae Lignum, as well as an image of the MYPplus capsule, are presented in Fig. 1.
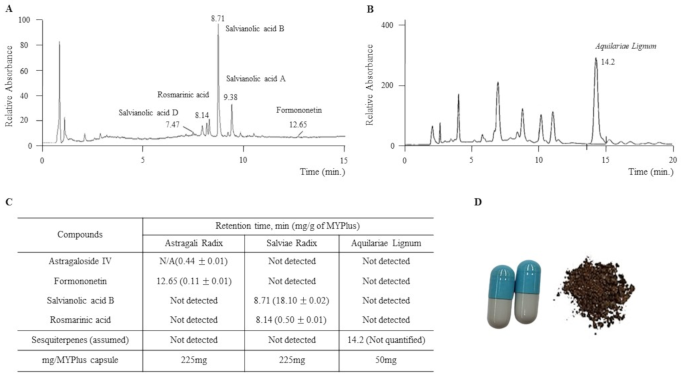
The intervention with MYPplus was decided based on the similarities in symptoms and pathophysiology between Long COVID and ME/CFS, along with the clinical efficacy of Myelophil (MYP) on patients with ME/CFS [17] and our previous results on Aquilariae Lignum improving neuroinflammation [19, 20].
Assessment of long COVID symptoms and safety
The primary outcome was the change in mKCFQ11 scores from baseline to 4 weeks, assessed using a self-rating questionnaire designed to measure the degree of fatigue. The mKCFQ11 is a modified Korean version of the Chalder Fatigue Scale (CFQ), the most commonly used fatigue-measuring scale, consisting of 11 questions (7 related to physical fatigue and 4 related to mental fatigue, with a total score ranging from 0 to 99) [22].
Secondary outcomes included changes in the Multidimensional Fatigue Inventory (MFI-20), the Visual Analogue Scale (VAS) for measuring general fatigue and brain fog symptoms, and the 12-item Short-Form Health Survey (SF-12) for assessing overall quality of life.
The safety of MYPplus was estimated through hematological analyses, which included liver and renal function tests, a complete blood count, and urinalysis. Adverse events were monitored through participant self-reports or identification of clinical signs and symptoms during the trial. We also followed the World Health Organization-Uppsala Monitoring Center (WHO-UMC) causality assessment system.
Determination of cytokines and cortisol concentration in plasma
To investigate the underlying mechanisms of Long COVID-related fatigue and assess the pharmacological effects of MYPplus, we measured three cytokines–tumor necrosis factor-α (TNF-α), transforming growth factor-β (TGF-β), and interferon-γ (IFN-γ)–which have been proposed to play a role in the persistence and recurrence of Long COVID symptoms [23, 24]. Additionally, we analyzed cortisol levels, which are known to be lower in Long COVID patients due to the presence of SARS-COV-2 antigens in the brain, as reduced cortisol levels can lead to increased brain inflammation [25]. Pre- and post-intervention comparisons were conducted to evaluate changes in these biomarkers. Blood was collected using a vacutainer system containing ethylenediamine tetraacetic acid (EDTA) under 12 h fasting condition, and plasma was isolated by centrifugation at 4 ℃ for 10 min. The plasma levels of TNF-α (cat. No. 555212), TGF-β (cat. No. 559119), and IFN-γ (cat. No. 555142) were determined using commercial ELISA kit (BD Biosciences, San Diego, CA, USA). Additionally, a cortisol parameter assay kit (cat. No. KGE008B, R&D system, Minneapolis, USA) was used to measure plasma cortisol levels. The absorbance was read at 450 and 570 nm with a spectrophotometer (Molecular Devices, Sunnyvale, CA, USA).
Statistical analysis
The analysis was conducted using the intention-to-treat (ITT) approach, which included all participants initially allocated and who received at least one dose of MYPplus in the statistical analysis. Safety analysis was based on the Safety analysis set (SS), comprising all randomized subjects who received at least one treatment dose. Changes in continuous variables, including mKCFQ11, MFI-20, VAS, SF-12, cytokines, and cortisol levels, were compared using a paired t-test. Additionally, Pearsons’ correlation analyses were performed between mKCFQ11 and secondary outcomes. Statistical significance was considered at a p-value < 0.05, and all statistical analyses were conducted using IBM SPSS Statistics software, version 23.
Results
Baseline characteristics and demographic data
Fifty subjects (18 males and 32 females) who met the inclusion criteria were enrolled, and all of them completed the 4-week study successfully. Data was collected in full, with no missing entries in assessment tools, and compliance with MYPplus capsule intake was high, as all participants consumed > 80% of the allocated capsules.
The mean age of the participants was 48.6 ± 14.1 years, and the duration of Long COVID averaged 20.3 ± 14.6 weeks. Baseline data were well balanced between males and females. Details regarding baseline demographics and clinical characteristics are shown in Table 1 and Supplementary Table 2.Table 1 Baseline characteristics of the participants
Changes in mKCFQ11 score
After 4 weeks of MYPplus intake, the mKCFQ11 score significantly decreased from 75.3 ± 10.9 to 40.5 ± 17.2 (p < 0.01), reflecting a 46% reduction in fatigue-related symptoms. Both physical (47.6 ± 7.5 to 25.9 ± 11.1, p < 0.01) and mental fatigue (27.7 ± 4.3 to 14.6 ± 7.0, p < 0.01) showed similar improvements (Fig. 2A; Table 2).
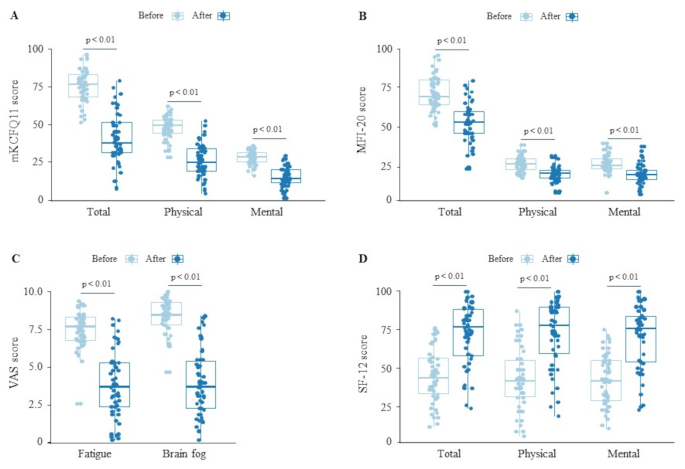
Table 2 Changes in fatigue assessments biomarkers after intervention
Changes in secondary outcomes
The MFI-20 score decreased by 26% (p < 0.01), while Fatigue VAS, Brain fog VAS, and SF-12 also showed significant improvements (Fig. 2B to D, Table 2). The MFI-20 and SF-12, analyzed separately for physical and mental aspects, also significant improvement, aligning with the results observed in mKCFQ11.
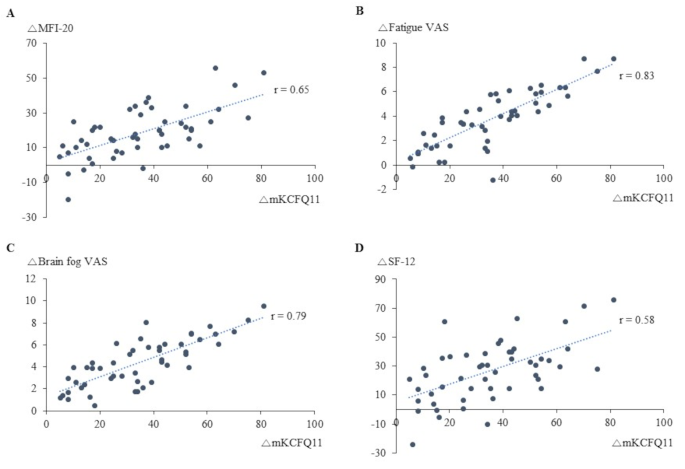
In an exploratory correlation analysis, mKCFQ11 changes showed strong positive correlations with Fatigue VAS (r = 0.83, p < 0.01) and Brain fog VAS (r = 0.79, p < 0.01), while moderate correlations were observed with MFI-20 (r = 0.65, p < 0.01) and SF-12 (r = 0.58, p < 0.01) (Fig. 3A to D).
Changes in cytokines and cortisol levels
Changes in cytokine concentrations, including TNF-α, TGF-β, and IFN- γ, did not show significant differences between pre- and post-administration of MYPplus. While plasma TGF-β levels showed a decreasing pattern after MYPplus administration, from 765.0 ± 1759.7 pg/mL to 242.0 ± 701.0 pg/mL (p = 0.07). Details regarding the cytokines and cortisol levels are provided in Table 2.
Safety
Adverse events were reported in two participants (4%); one experienced mild diarrhea, while the other reported mild diarrhea accompanied by abdominal pain. Both adverse events resolved within a week without the need for any specific treatment.
All hematological and biochemical parameters, including complete blood count, liver and renal function tests, and urinalysis, remained within acceptable ranges both at baseline and after the 4 weeks of MYPplus administration (data not shown, see Additional file 1).
Discussion
The COVID-19 pandemic has led to the emergence of Long COVID, also known as Post-COVID Conditions (PCC), characterized by persistent symptoms such as fatigue (53.1%), reduced quality of life (44.1%), and dyspnea (43.4%), which can last for months or even years after SARS-CoV-2 infection [26]. Long COVID imposes a significant disease burden, resulting in over 80 disability-adjusted life years (DALYs) per 1,000 individuals among non-hospitalized patients–surpassing the burden of heart disease (52 DALYs) and cancer (50 DALYs) [27, 28]. Despite its widespread impact, there is currently no approved treatment; management relies on symptomatic care, self-monitoring, and rehabilitation [29].
Notably, the hallmark symptoms of Long COVID, such as fatigue and cognitive dysfunction, closely resemble those observed in ME/CFS, with approximately 50% of Long COVID patients meeting ME/CFS diagnostic criteria [30, 31]. Moreover, symptom severity–particularly in terms of fatigue, brain fog, and orthostatic intolerance–appears comparable between the two conditions [32]. We herein investigated the effects of MYPplus. a modified MYP formulation consisting of a 30% ethanol extract of Astragali Radix and Salviae Radix, supplemented with Aquilariae Lignum, which has demonstrated neuroinflammation-reducing properties in prior studies [19, 20]. After four weeks of MYPplus treatment, fatigue levels significantly improved, with a 46% reduction in mKCFQ11 score and a 49% reduction in Fatigue VAS (Fig. 2A and C; Table 2). Brain fog VAS decreased by 52%, and quality of life, as measured by SF-12, improved by 59% (Fig. 2C and D; Table 2). These measures exhibited strong positive correlations (Fig. 3A to D). However, while mKCFQ11 and Fatigue VAS showed notable reductions, the MFI-20 score improvement was comparatively smaller (26%), possibly due to differences in sensitivity between fatigue-measurement tools. When we compared two scales in the same population, the mKCFQ11 more sensitively reflected fatigue scores in subjects with any spectrum of fatigue levels than the MFI-20 tool [33].
These findings suggest MYPplus as a potential therapeutic option for Long COVID-related fatigue and brain fog. Brain fog, encompassing cognitive difficulties such as dissociation and forgetfulness [34], is believed to stem from neuroinflammation [35]. Microglial activation and elevated cytokine levels disrupt cognitive regulation via pathways such as the HPA axis and the kynurenine pathway [36]. Our previous studies have demonstrated the neuroinflammation-modulating effects of Aquilariae Lignum and MYP [19, 20, 37], supporting the observed clinical improvements in brain fog symptoms.
From a pathophysiological perspective, studies have highlighted several potential mechanisms underlying Long COVID, including persisting viral reservoirs, sustained inflammation, host microbiome factors, and ongoing autoimmune responses [38]. One critical factor is the inhibition of nuclear factor erythroid 2-related factor 2 (Nrf2) by SARS-CoV-2 infection, which disrupts its role in suppressing pro-inflammatory cytokine expression and mitigating oxidative stress [9, 39]. Another contributor is prostaglandins (PGs), which act as cytokine amplifiers by triggering the release of pro-inflammatory cytokines, thereby promoting chronic inflammation [8]. Consequently, prolonged symptoms of Long COVID have been linked to persistent alterations in cytokine levels, particularly IL-6, TNF-α, and IFN-γ [23, 40]. Additionally, genetic polymorphisms in IL-6, TNF-α, and IFN-γ have been associated with post-viral fatigue and neurocognitive impairments [41].
To gain mechanical insights into MYPplus, we assessed changes in plasma levels of TNF-α, IFN-γ, and TGF-β, along with cortisol (Table 2). No significant change was observed in TNF-α levels following treatment, although some cases showed substantial variation. Four patients exhibited highly elevated TNF-α levels accompanied by notable fatigue before treatment, which subsequently decreased after MYPplus administration (from 54.4 ± 87.8 pg/mL to 31.2 ± 50.3 pg/mL, p = 0.068, data not shown). Studies have reported both decreased and elevated IFN-γ levels in patients with Long COVID, suggesting either immune exhaustion or chronic inflammation, respectively [42, 43]. A previous study demonstrated that the resolution of Long COVID symptoms coincided with normalization of IFN-γ levels [44]. In the present study, participants exhibited notable variations in IFN-γ levels, and while MYPplus administration showed a slight upward trend without statistical significance (from 11.7 ± 65.6 pg/mL to 33.6 ± 214.3 pg/mL, p = 0.30). This result suggests that MYPplus intake may have contributed to enhanced immune activity and improvements in fatigue and/or brain fog among patients with Long COVID.
Notably, TGF-β levels showed a borderline significant decrease (p = 0.07) following MYPplus treatment (Table 2). Elevated TGF-β has been implicated in the persistent sequelae of COVID-19 and has been proposed as a biomarker for Long COVID and disease severity [24, 45, 46]. Furthermore, TGF-β levels correlate with the severity of ME/CFS [47, 48], which aligns with our findings of TGF-β activation in all brain regions of adrenalectomized mice exhibiting ME/CFS-like symptoms [49]. Additionally, our preclinical research demonstrated that MYP modulates TGF-β activity in key brain regions, including the hippocampus, in a reserpine-induced ME/CFS model [50]. Along with alterations in cytokine levels, cortisol is also known to influence the pathology of Long COVID through various pathways, including stress response and immune regulation [51]. A previous study reported that Long COVID patients tend to have cortisol levels approximately 50% lower than those of matched controls, with this deficiency persisting beyond one year post-infection due to the absence of a compensatory increase in adrenocorticotropic hormone (ACTH) [45]. Conversely, another study found elevated cortisol levels in some Long COVID patients [52]. However, all participants in the present study had cortisol levels within the normal range, which may have influenced our results. As expected, no significant changes were observed in cortisol levels, although a slight decreasing trend was noted after treatment (from 120.7 ± 30.1 ng/mL to 110.7 ± 61.8 ng/mL, p = 0.27).
These results presented above indicate the potential of MYPplus in managing fatigue symptoms associated with Long COVID. However, this study has some limitations, most notably its observational nature and relatively small sample size, which raises the possibility of bias. In terms of safety, no significant issues were observed throughout the trial period, as measured by hematological, biochemical parameters, and urinalysis. Two participants (4%) experienced mild diarrhea, which resolved within a week without specific treatment.
Conclusions
This observational study provides preliminary clinical evidence suggesting that MYPplus may have pharmacological potential for managing fatigue and/or brain fog symptoms associated with Long COVID. Given these findings, it is imperative to conduct further well-designed studies with a placebo-controlled design and a larger participant pool in the future.
