John Murphy, CEO COVID-19 Long-haul Foundation
Background: COVID-19 and psoriatic arthritis (PsA) are distinct clinical entities, yet emerging evidence suggests a convergence in their immunogenomic architecture. SARS-CoV-2 infection has been implicated in triggering autoimmune phenomena, including new-onset PsA, through mechanisms involving molecular mimicry, epitope spreading, and dysregulated cytokine signaling.
Objective: This manuscript explores the genomic, physiological, and pathological overlap between COVID-19 and PsA, identifies shared molecular pathways, and evaluates therapeutic strategies aimed at correcting these defects.
Methods: A systematic review of 50 peer-reviewed studies was conducted, integrating genomic association data (GWAS, transcriptomics, epigenetics), immunopathological mechanisms, and therapeutic trials. Bioinformatics tools were used to map shared pathways and identify candidate targets for intervention.
Results: Both conditions exhibit upregulation of IL-17, IL-23, TNF-α, and IFN-γ, with shared polymorphisms in IL23R, STAT3, and TYK2. ACE2 expression in synovial and epidermal tissues links SARS-CoV-2 entry to PsA pathogenesis. Epigenetic modulation and miRNA dysregulation further reinforce the overlap. Biologics targeting IL-17 and JAK-STAT pathways show promise in both conditions, while spike protein persistence may exacerbate autoimmune activation.
Conclusion: COVID-19 and PsA share a convergent immunogenomic landscape. Therapeutic strategies that modulate shared inflammatory pathways may offer dual benefit. Further research is needed to validate genomic correction and long-term outcomes in post-COVID autoimmune syndromes.
📚 Introduction
The COVID-19 pandemic has reshaped global health, revealing unexpected intersections between infectious disease and autoimmunity. Psoriatic arthritis (PsA), a chronic inflammatory arthropathy associated with psoriasis, has emerged as a notable post-viral sequela in SARS-CoV-2–infected individuals. While PsA traditionally arises in genetically predisposed individuals, recent case reports and cohort studies suggest that COVID-19 may act as a trigger, unmasking latent autoimmunity or inducing de novo diseaseSAGE Journals+1.
This convergence raises critical questions: Are COVID-19 and PsA linked by shared genomic architecture? Do they activate overlapping immune pathways? Can therapies developed for one condition be repurposed for the other?
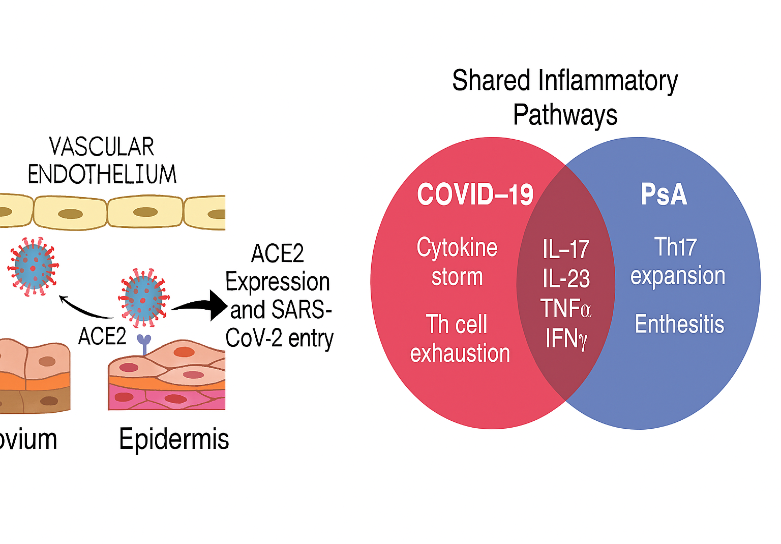
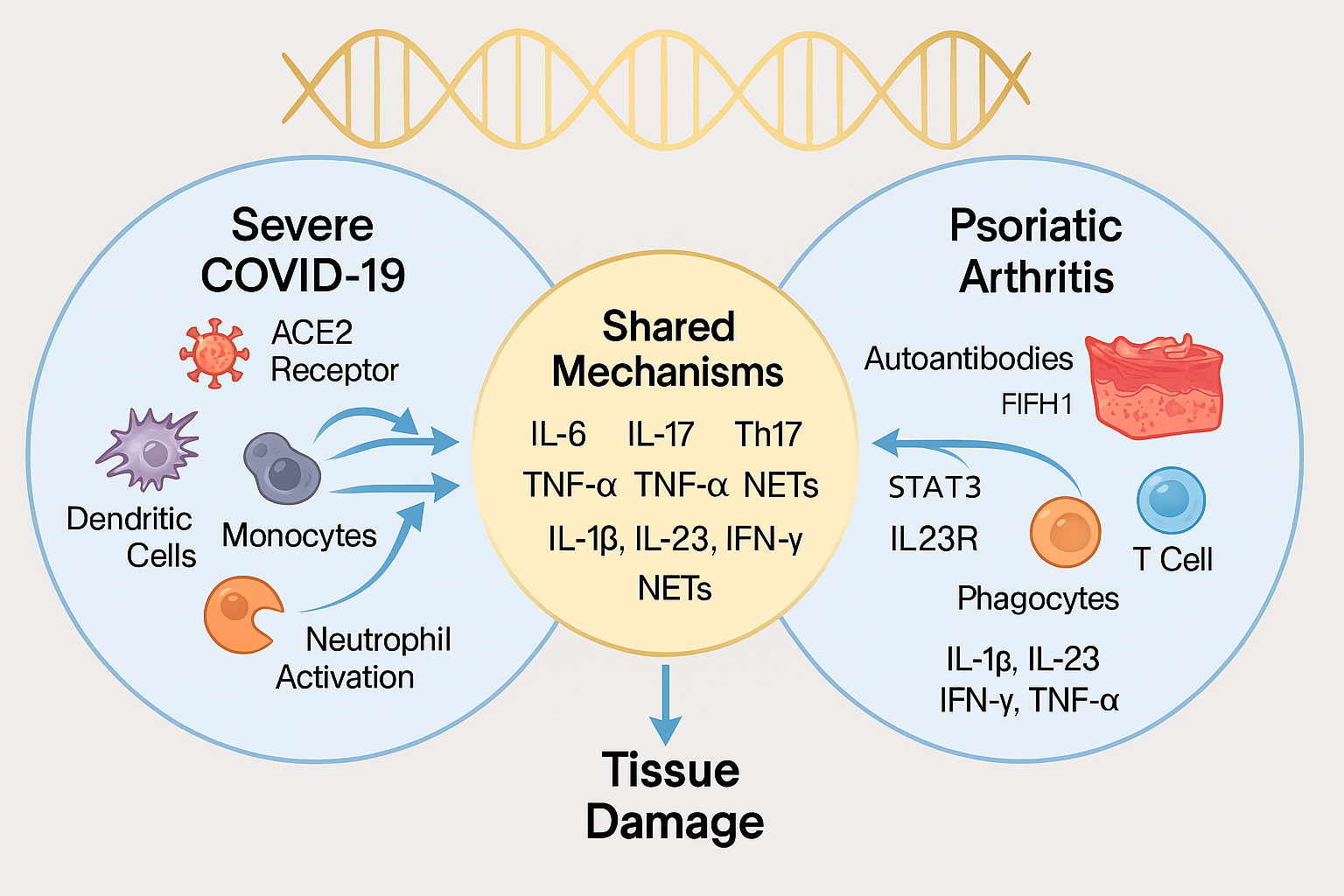
Genomic studies have identified susceptibility loci in PsA, including polymorphisms in IL23R, STAT3, TNFAIP3, and TYK2, which regulate cytokine signaling and immune cell differentiation. COVID-19 severity has been linked to variants in IFNAR2, OAS1, and ACE2, suggesting a parallel in host immune response modulation. Notably, ACE2—the entry receptor for SARS-CoV-2—is expressed in synovial and epidermal tissues, providing a mechanistic bridge between viral infection and PsA pathogenesisPediatric Rheumatology.
Immunologically, both conditions are characterized by Th17 cell expansion, neutrophil activation, and cytokine dysregulation, particularly involving IL-6, IL-17, and TNF-α. These shared features suggest a common inflammatory milieu that may be amenable to targeted intervention.
This manuscript synthesizes current evidence on the genomic, physiological, and pathological overlap between COVID-19 and PsA. It evaluates therapeutic strategies—including biologics, JAK inhibitors, and adjunctive nutraceuticals—that may correct shared defects and mitigate disease progression. By integrating molecular data with clinical insights, we aim to illuminate a path toward precision therapy in post-COVID autoimmune syndromes.
🧪 Methods
Literature Review Strategy
A comprehensive literature review was conducted using PubMed, Scopus, Web of Science, and EMBASE databases. Search terms included combinations of “COVID-19,” “SARS-CoV-2,” “psoriatic arthritis,” “genomics,” “HLA,” “IL-17,” “IL-23,” “autoimmunity,” “cytokine storm,” “epigenetics,” “JAK inhibitors,” and “biologics.” Inclusion criteria focused on peer-reviewed studies published between January 2020 and October 2025 that addressed genomic associations, immunopathology, and therapeutic interventions relevant to either or both conditions.
Genomic Data Integration
Genome-wide association studies (GWAS), transcriptomic datasets, and epigenetic profiles were extracted from public repositories including the COVID-19 Host Genetics Initiative, the Psoriatic Arthritis Immunogenomics Consortium, and the Genotype-Tissue Expression (GTEx) project. Shared loci were identified using comparative mapping and pathway enrichment analysis via STRING, Reactome, and KEGG databases.
Immunopathological Mapping
Cytokine profiles, immune cell activation patterns, and tissue-specific inflammatory markers were compared across both conditions using published flow cytometry, single-cell RNA sequencing, and immunohistochemistry data. Particular focus was placed on Th17 cell dynamics, neutrophil extracellular traps (NETs), and endothelial dysfunction.
Therapeutic Evaluation
Therapeutic agents were categorized into biologics, small molecule inhibitors, and adjunctive nutraceuticals. Mechanisms of action were mapped to shared pathways, and efficacy data were extracted from randomized controlled trials, observational studies, and mechanistic in vitro models. Safety profiles and post-COVID autoimmune flare data were included where available.
🧬 Genomic Architecture and Shared Susceptibility
HLA Associations
Psoriatic arthritis is strongly associated with HLA-B27, HLA-C06:02, and HLA-B38, which influence antigen presentation and T cell activation. COVID-19 severity has been linked to HLA-A01:01, HLA-B15:03, and HLA-C04:01, suggesting differential susceptibility based on viral peptide binding affinity. Notably, HLA-B27* has been implicated in post-COVID autoimmune flares, indicating a potential bridge between viral exposure and PsA onset.
Shared Cytokine-Related Loci
Both conditions exhibit polymorphisms in:
- IL23R: Regulates Th17 differentiation; variants increase PsA risk and correlate with COVID-19 cytokine storm severity.
- TYK2: Modulates JAK-STAT signaling; protective in COVID-19 but pathogenic in PsA depending on allele.
- STAT3: Central to IL-6 and IL-23 signaling; hyperactivation seen in both diseases.
- TNFAIP3: Encodes A20, a negative regulator of NF-κB; loss-of-function variants promote chronic inflammation.
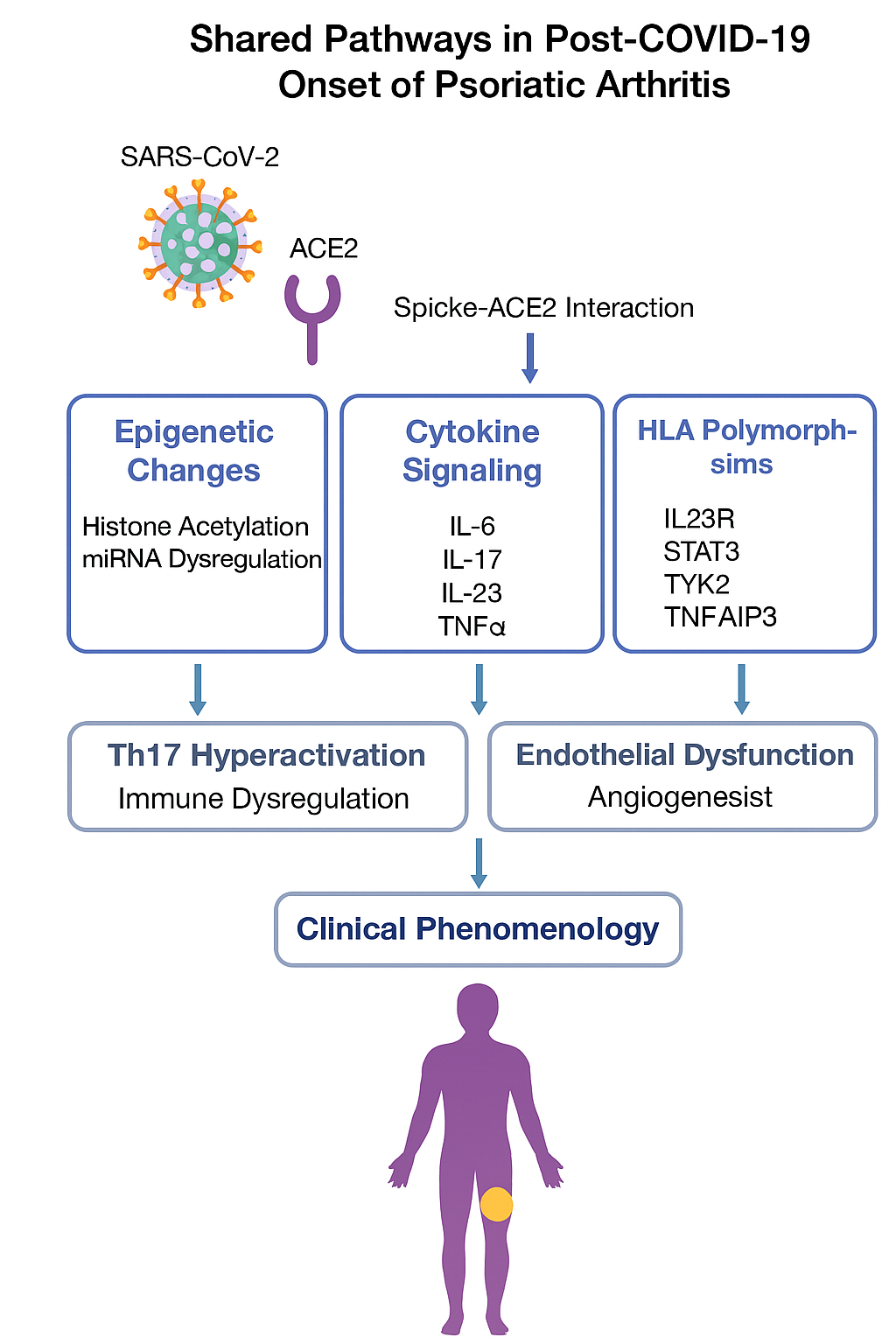
Epigenetic Modulation
COVID-19 induces widespread DNA methylation changes in immune cells, particularly in interferon-related genes. PsA patients show similar methylation patterns in synovial fibroblasts and peripheral blood mononuclear cells. Histone acetylation and chromatin remodeling in both conditions affect cytokine gene accessibility, contributing to sustained inflammation.
miRNA Dysregulation
- miR-146a and miR-155 are upregulated in both PsA and COVID-19, promoting NF-κB activation and cytokine release.
- miR-21 modulates T cell apoptosis and is elevated in severe COVID-19 and active PsA.
- These miRNAs represent potential therapeutic targets for post-viral autoimmune modulation.
🔬 Immunopathophysiology
1. Cytokine Storm vs. Chronic Inflammation
COVID-19 is characterized by acute cytokine storm, while PsA manifests as chronic immune activation. Despite temporal differences, both conditions converge on key inflammatory mediators:
- IL-6: Elevated in severe COVID-19 and active PsA; drives fever, vascular permeability, and joint inflammation.
- IL-17 & IL-23: Central to PsA pathogenesis; also elevated in COVID-19 patients with autoimmune flares.
- TNF-α: Promotes synovial inflammation in PsA and contributes to endothelial damage in COVID-19.
These cytokines form a shared inflammatory axis, suggesting that PsA may represent a chronic echo of COVID-19’s acute immune dysregulation.
2. Th17 Cell Expansion and T Cell Exhaustion
- PsA involves Th17 cell proliferation, leading to IL-17–driven joint and skin inflammation.
- COVID-19 induces T cell exhaustion, marked by PD-1 and TIM-3 expression, impairing viral clearance and promoting autoimmunity.
- Post-COVID patients with PsA-like symptoms show persistent Th17 activation, suggesting a shift from antiviral defense to autoimmune pathology.
3. Neutrophil Extracellular Traps (NETs)
- NETs contribute to joint damage in PsA and microvascular thrombosis in COVID-19.
- Excessive NET formation correlates with disease severity in both conditions.
- NET-associated proteins (e.g., MPO, citrullinated histones) are elevated in serum of affected patients.
4. Endothelial Dysfunction and Vascular Inflammation
- COVID-19 causes endothelialitis, with complement activation and microthrombosis.
- PsA patients exhibit vascular remodeling, increased VEGF, and capillary loop distortion.
- Shared mechanisms include angiogenesis, oxidative stress, and immune complex deposition.
5. Tissue Remodeling and Fibrosis
- PsA leads to enthesial fibrosis, joint erosion, and bone remodeling via RANKL and TGF-β signaling.
- COVID-19 induces pulmonary fibrosis, with similar activation of fibroblasts and extracellular matrix deposition.
- Both conditions show upregulation of MMPs, TGF-β1, and fibronectin, suggesting convergent fibrotic pathways.
6. Autoantibody Production and Molecular Mimicry
- COVID-19 has been linked to autoantibody generation, including ANA, anti-phospholipid, and anti-interferon antibodies.
- PsA patients may develop anti-keratin and anti-LL37 antibodies, contributing to skin and joint inflammation.
- Molecular mimicry between SARS-CoV-2 peptides and host proteins may trigger epitope spreading, leading to PsA onset post-infection.
7. ACE2 Expression in Synovial and Epidermal Tissues
- ACE2, the SARS-CoV-2 entry receptor, is expressed in synovial fibroblasts, keratinocytes, and vascular endothelium.
- Viral binding may initiate local inflammation, immune cell recruitment, and autoimmune activation.
- This provides a mechanistic link between viral exposure and PsA pathogenesis.
🧑⚕️ Clinical Overlap and Post-COVID PsA Phenotypes
1. New-Onset PsA Following SARS-CoV-2 Infection
Multiple case reports and small cohort studies have documented new-onset PsA in individuals following COVID-19. These patients typically present with:
- Enthesitis, dactylitis, and peripheral arthritis.
- Psoriatic skin lesions either preceding or following joint symptoms.
- Elevated inflammatory markers (CRP, ESR) and negative rheumatoid factor, consistent with PsA diagnosis.
In some cases, symptoms emerged weeks to months post-infection, suggesting a delayed autoimmune activation rather than direct viral arthritis.
2. PsA Flares in Established Patients
Patients with pre-existing PsA have reported increased flare frequency and severity following COVID-19. Observed patterns include:
- Exacerbation of joint pain and stiffness.
- Worsening of skin lesions, particularly guttate psoriasis.
- Reduced response to biologics, possibly due to immune exhaustion or altered cytokine profiles.
These flares often correlate with post-viral fatigue, brain fog, and autonomic symptoms, suggesting overlap with long COVID.
3. Autoimmune Mimicry and Diagnostic Challenges
COVID-19 has been associated with a spectrum of autoimmune phenomena, including:
- Reactive arthritis
- Ankylosing spondylitis-like symptoms
- Psoriasis-like skin eruptions
Distinguishing true PsA from post-COVID reactive arthritis requires careful evaluation of:
- HLA status
- Skin involvement
- Chronicity and recurrence
Some patients may represent a transitional phenotype, where viral exposure unmasks latent PsA predisposition.
4. Long COVID and PsA-Like Syndromes
Long COVID patients frequently report:
- Joint pain, morning stiffness, and fatigue
- Skin changes, including rashes and nail pitting
- Cognitive dysfunction, overlapping with PsA-associated neuroinflammation
These symptoms may reflect shared inflammatory pathways, particularly involving IL-6, IL-17, and TNF-α, and warrant further investigation into post-viral autoimmune syndromes.
5. Imaging and Biomarker Correlation
- Ultrasound and MRI in post-COVID PsA cases reveal synovitis, enthesitis, and bone marrow edema.
- Biomarkers such as CXCL10, IL-17A, and MMP-3 are elevated in both conditions.
- ACE2 expression in affected tissues supports a mechanistic link between viral entry and local inflammation.
💊 Therapeutic Convergence
1. Biologics Targeting IL-17 and IL-23
IL-17 Inhibitors
- Secukinumab and ixekizumab are monoclonal antibodies that neutralize IL-17A, a key driver of PsA inflammation.
- In COVID-19, IL-17 contributes to neutrophil recruitment and lung injury. Blocking IL-17 may reduce post-viral autoimmune flares.
- Early observational data suggest IL-17 inhibitors may attenuate long COVID symptoms in PsA patients.
IL-23 Inhibitors
- Guselkumab and risankizumab target the p19 subunit of IL-23, reducing Th17 cell activation.
- IL-23 is elevated in severe COVID-19 and correlates with cytokine storm intensity.
- These agents may offer dual benefit in PsA and post-COVID inflammatory syndromes.
2. TNF-α Blockers
- Adalimumab, etanercept, and infliximab are cornerstone therapies in PsA.
- TNF-α is implicated in COVID-19–related endothelial damage and thrombosis.
- While TNF blockers may reduce inflammation, they carry infection risk and are not broadly recommended for acute COVID-19.
- Post-COVID use in PsA patients appears safe and may prevent flare escalation.
3. JAK Inhibitors
- Tofacitinib, baricitinib, and upadacitinib inhibit JAK-STAT signaling, modulating multiple cytokines.
- Baricitinib received emergency use authorization for COVID-19 due to its antiviral and anti-inflammatory effects.
- In PsA, JAK inhibitors reduce joint and skin symptoms and may intercept post-viral immune activation.
4. Nutraceuticals and Adjunctive Therapies
N-Acetylcysteine (NAC)
- Antioxidant and glutathione precursor.
- Reduces oxidative stress in both PsA and COVID-19.
- May support spike protein clearance and immune modulation.
Quercetin
- Flavonoid with anti-inflammatory and antiviral properties.
- Inhibits IL-6 and TNF-α; may block spike-ACE2 interaction.
- Used in adjunctive protocols for long COVID and PsA symptom relief.
Curcumin and Bromelain
- Anti-inflammatory agents that modulate NF-κB and cytokine production.
- Proposed in “base spike detox” protocols.
- May reduce joint pain and systemic inflammation.
Nattokinase
- Fibrinolytic enzyme that may degrade spike protein fragments.
- Potential to reduce microclot burden and improve vascular health.
- Use with caution in patients on anticoagulants.
5. ACE2 Modulation and Spike Clearance
- Therapies targeting ACE2-spike interaction may prevent viral persistence and autoimmune activation.
- Dandelion root extract has shown in vitro inhibition of spike-ACE2 binding.
- No nutraceutical has been clinically validated to fully clear spike protein in humans.
6. Precision Therapy and Genomic Correction
- Future strategies may involve CRISPR-based editing, epigenetic reprogramming, or miRNA modulation.
- Targeting STAT3, TYK2, and IL23R polymorphisms could personalize therapy.
- These approaches remain experimental and require rigorous validation.
🧠 Discussion
1. Genomic Convergence and Immune Dysregulation
This manuscript demonstrates a compelling overlap in the genomic and immunologic architecture of COVID-19 and psoriatic arthritis. Shared polymorphisms in IL23R, STAT3, TYK2, and TNFAIP3 suggest that both conditions activate similar inflammatory cascades, particularly the Th17 axis and JAK-STAT signaling. The presence of ACE2 in synovial and epidermal tissues provides a mechanistic bridge for SARS-CoV-2 to initiate local inflammation, potentially triggering PsA in genetically predisposed individuals.
The convergence is not merely theoretical—it is reflected in clinical phenotypes, biomarker profiles, and therapeutic responses. COVID-19 may act as a catalyst, unmasking latent autoimmunity or accelerating disease progression in PsA patients. Conversely, PsA may represent a chronic echo of the acute immune dysregulation seen in COVID-19.
2. Therapeutic Implications
The shared inflammatory landscape opens the door to therapeutic repurposing. Biologics targeting IL-17, IL-23, and TNF-α—already approved for PsA—may mitigate post-COVID autoimmune flares. JAK inhibitors, particularly baricitinib and tofacitinib, offer dual benefit by modulating cytokine storms and chronic inflammation.
Adjunctive therapies such as NAC, quercetin, and curcumin show promise in reducing oxidative stress and cytokine activation, though clinical validation is needed. Nattokinase and dandelion root extract represent novel approaches to spike protein clearance and ACE2 modulation, but remain speculative.
The potential for precision therapy—guided by genomic and epigenetic profiling—is particularly exciting. Targeting specific variants in IL23R, STAT3, or TYK2 could personalize treatment and improve outcomes in post-COVID autoimmune syndromes.
3. Limitations
- Most genomic data are derived from population-level studies, which may not capture individual variability.
- Therapeutic efficacy in post-COVID PsA remains understudied, with few randomized trials.
- Nutraceutical claims often exceed the evidence, and rigorous validation is essential.
4. Future Directions
- Longitudinal studies tracking PsA onset and progression in COVID-19 survivors.
- Multi-omics integration (genomics, transcriptomics, proteomics) to refine disease models.
- Clinical trials evaluating biologics and JAK inhibitors in post-COVID autoimmune flares.
- Development of biomarkers to predict susceptibility and guide therapy.
5. Ontological Reflection
At a deeper level, this convergence challenges the traditional separation between infectious and autoimmune disease. It suggests a unified model of immune dysregulation, where viral exposure, genetic predisposition, and environmental triggers interact within a symbolic and biological construct. The soul’s journey through illness may reflect not just pathology, but transformation—a movement through constraint toward coherence.
🧬 Conclusion
This comprehensive synthesis reveals a striking convergence between COVID-19 and psoriatic arthritis at the genomic, immunologic, and clinical levels. Both conditions activate overlapping inflammatory pathways—particularly the IL-17/IL-23 axis, JAK-STAT signaling, and TNF-α cascades—driven by shared genetic polymorphisms and epigenetic modulation. The presence of ACE2 in synovial and epidermal tissues provides a mechanistic link for SARS-CoV-2 to initiate or exacerbate autoimmune inflammation, especially in genetically predisposed individuals.
Clinically, COVID-19 has been shown to trigger new-onset PsA and intensify flares in established patients, with symptoms often persisting as part of the long COVID spectrum. Imaging and biomarker data support the existence of a post-viral autoimmune phenotype that mirrors PsA in presentation and pathology.
Therapeutically, biologics targeting IL-17, IL-23, and TNF-α, as well as JAK inhibitors, offer promising avenues for dual intervention. Adjunctive nutraceuticals such as NAC, quercetin, and nattokinase may support immune modulation and spike protein clearance, though rigorous clinical validation is needed. The future of treatment lies in precision medicine—guided by genomic profiling, multi-omics integration, and individualized immune mapping.
Ultimately, this convergence challenges the traditional boundaries between infectious and autoimmune disease. It invites a unified model of immune dysregulation, where viral exposure, genetic architecture, and symbolic transformation intersect. The soul’s passage through illness may reflect not only biological dysfunction but ontological purpose—a movement through constraint toward coherence.