Scientists debate evidence for a micro-clot hypothesis that has some people pursuing potentially risky treatments
Authors: Cassandra Willyard Nature 608, 662-664 (2022)doi: https://doi.org/10.1038/d41586-022-02286-7
When Lara Hawthorne, an illustrator in Bristol, UK, began developing strange symptoms after having COVID-19, she hoped that they weren’t due to the virus. Her initial illness had been mild. “I’ve been triple vaccinated. I felt quite protected,” she says. But months later, she was still sick with a variety of often debilitating symptoms: earaches, tinnitus, congestion, headaches, vertigo, heart palpitations, muscle pain and more. On some days, Hawthorne felt so weak that she could not get out of bed. When she finally saw her physician, the diagnosis was what she had been dreading: long COVID.
Unable to find relief, she became increasingly desperate. After reading an opinion piece in The Guardian newspaper about how blood clots might be to blame for long COVID symptoms, Hawthorne contacted a physician in Germany who is treating people with blood thinners and a procedure to filter the blood. She hasn’t heard back yet — rumour has it that people stay on the waiting list for months — but if she has the opportunity to head there for these unproven treatments, she probably will. “I don’t want to wait on my health when I’m feeling so dreadful,” she says.
Researchers are baffled by long COVID: hundreds of studies have tried to unpick its mechanism, without much success. Now some scientists, and an increasing number of people with the condition, have been lining up behind the as-yet-unproven hypothesis that tiny, persistent clots might be constricting blood flow to vital organs, resulting in the bizarre constellation of symptoms that people experience.
Heart disease after COVID: what the data say
Proponents of the idea (#teamclots, as they sometimes refer to themselves on Twitter) include Etheresia Pretorius, a physiologist at Stellenbosch University in South Africa, and Douglas Kell, a systems biologist at the University of Liverpool, UK, who led the first team to visualize micro-clots in the blood of people with long COVID. They say that the evidence implicating micro-clots is undeniable, and they want trials of the kinds of anticoagulant treatment that Hawthorne is considering. Pretorius penned the Guardian article that caught Hawthorne’s attention.
But many haematologists and COVID-19 researchers worry that enthusiasm for the clot hypothesis has outpaced the data. They want to see larger studies and stronger causal evidence. And they are concerned about people seeking out unproven, potentially risky treatments.
When it comes to long COVID, “we’ve now got little scattered of bits of evidence”, says Danny Altmann, an immunologist at Imperial College London. “We’re all scuttling to try and put it together in some kind of consensus. We’re so far away from that. It’s very unsatisfying.”
Cascade of clots
Pretorius and Kell met about a decade ago. Pretorius had been studying the role of iron in clotting and neglected to cite some of Kell’s research. When he reached out, they began chatting. “We had a Skype meeting and then we decided to work together,” Pretorius says. They observed odd, dense clots that resist breaking down for years in people with a variety of diseases. The research led them to develop the theory that some molecules — including iron, proteins or bits of bacterial cell wall — might trigger these abnormal clots.
Blood clotting is a complex process, but one of the key players is a cigar-shaped, soluble protein called fibrinogen, which flows freely in the bloodstream. When an injury occurs, cells release the enzyme thrombin, which cuts fibrinogen into an insoluble protein called fibrin. Strands of fibrin loop and criss-cross, creating a web that helps to form a clot and stop the bleeding.
Under a microscope, this web typically resembles “a nice plate of spaghetti”, Kell says. But the clots that the team has identified in many inflammatory conditions look different. They’re “horrible, gunky, dark”, Kell says, “such as you might get if you half-boiled the spaghetti and let it all stick together.” Research by Kell, Pretorius and their colleagues suggests that the fibrin has misfolded1, creating a gluey, ‘amyloid’ version of itself. It doesn’t take much misfolding to seed disaster, says Kell. “If the first one changes its conformation, all the others have to follow suit”, much like prions, the infectious misfolded proteins that cause conditions such as Creutzfeldt–Jakob disease.
Long-COVID treatments: why the world is still waiting
Pretorius first saw these strange, densely matted clots in the blood of people with a clotting disorder2, but she and Kell have since observed the phenomenon in a range of conditions1 — diabetes, Alzheimer’s disease and Parkinson’s disease, to name a few. But the idea never gained much traction, until now.
When the pandemic hit in 2020, Kell and Pretorius applied their methods almost immediately to people who had been infected with SARS-CoV-2. “We thought to look at clotting in COVID, because that is what we do,” Pretorius says. Their assay uses a special dye that fluoresces when it binds to amyloid proteins, including misfolded fibrin. Researchers can then visualize the glow under a microscope. The team compared plasma samples from 13 healthy volunteers, 15 people with COVID-19, 10 people with diabetes and 11 people with long COVID3. For both long COVID and acute COVID-19, Pretorius says, the clotting “was much more than we have previously found in diabetes or any other inflammatory disease”. In another study4, they looked at the blood of 80 people with long COVID and found micro-clots in all of the samples.
So far, Pretorius, Kell and their colleagues are the only group that has published results on micro-clots in people with long COVID.
But in unpublished work, Caroline Dalton, a neuroscientist at Sheffield Hallam University’s Biomolecular Sciences Research Centre, UK, has replicated the results. She and her colleagues used a slightly different method, involving an automated microscopy imaging scanner, to count the number of clots in blood. The team compared 3 groups of about 25 individuals: people who had never knowingly had COVID-19, those who had had COVID-19 and recovered, and people with long COVID. All three groups had micro-clots, but those who had never had COVID-19 tended to have fewer, smaller clots, and people with long COVID had a greater number of larger clots. The previously infected group fell in the middle. The team’s hypothesis is that SARS-CoV-2 infection creates a burst of micro-clots that go away over time. In individuals with long COVID, however, they seem to persist.
Dalton has also found that fatigue scores seem to correlate with micro-clot counts, at least in a few people. That, says Dalton, “increases confidence that we are measuring something that is mechanistically linked to the condition”.
In many ways, long COVID resembles another disease that has defied explanation: chronic fatigue syndrome, also known as myalgic encephalomyelitis (ME/CFS). Maureen Hanson, who directs the US National Institutes of Health (NIH) ME/CFS Collaborative Research Center at Cornell University in Ithaca, New York, says that Pretorius and Kell’s research has renewed interest in a 1980s-era hypothesis about abnormal clots contributing to symptoms. Pretorius, Kell and colleagues found amyloid clots in the blood of people with ME/CFS, but the amount was much lower than what they’ve found in people with long COVID5. So clotting is probably only a partial explanation for ME/CFS, Pretorius says.
Micro-clot mysteries
Where these micro-clots come from isn’t entirely clear. But Pretorius and Kell think that the spike protein, which SARS-CoV-2 uses to enter cells, might be the trigger in people with long COVID. When they added the spike protein to plasma from healthy volunteers in the laboratory, that alone was enough to prompt formation of these abnormal clots6.
Bits of evidence hint that the protein might be involved. In a preprint7 posted in June, researchers from Harvard University in Boston, Massachusetts, reported finding the spike protein in the blood of people with long COVID. Another paper8 from a Swedish group showed that certain peptides in the spike can form amyloid strands on their own, at least in a test tube. It’s possible that these misfolded strands provide a kind of template, says Sofie Nyström, a protein chemist at Linköping University in Sweden and an author of the paper.
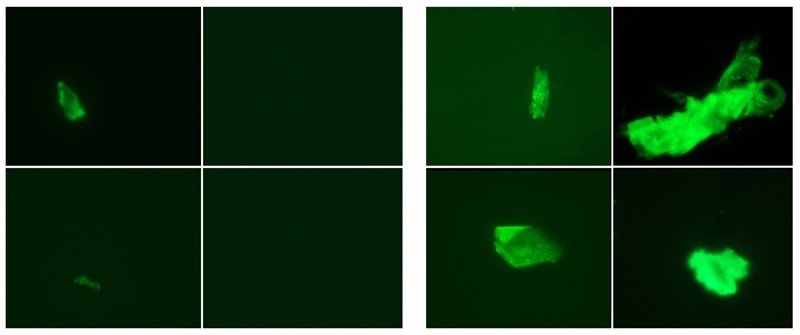
A California-based group found that fibrin can actually bind to the spike. In a 2021 preprint9, it reported that when the two proteins bind, fibrin ramps up inflammation and forms clots that are harder to degrade. But how all these puzzle pieces fit together isn’t yet clear.
If the spike protein is the trigger for abnormal clots, that raises the question of whether COVID-19 vaccines, which contain the spike or instructions for making it, can induce them as well. There’s currently no direct evidence implicating spike from vaccines in forming clots, but Pretorius and Kell have received a grant from the South African Medical Research Council to study the issue. (Rare clotting events associated with the Oxford–AstraZeneca vaccine are thought to happen through a different mechanism (Nature 596, 479–481; 2021).)
Raising safety concerns about the vaccines can be uncomfortable, says Per Hammarström, a protein chemist at Linköping University and Nyström’s co-author. “We don’t want to be over-alarmist, but at the same time, if this is a medical issue, at least in certain people, we have to address that.” Gregory Poland, director of the Mayo Clinic’s vaccine research group in Rochester, Minnesota, agrees that it’s an important discussion. “My guess is that spike and the virus will turn out to have a pretty impressive list of pathophysiologies,” he says. “How much of that may or may not be true for the vaccine, I don’t know.”
Dearth of data
Many researchers find it plausible and intriguing that micro-clots could be contributing to long COVID. And the hypothesis does seem to fit with other data that have emerged on clotting. Researchers already know that people with COVID-19, especially severe disease, are more likely to develop clots. The virus can infect cells lining the body’s 100,000 kilometres of blood vessels, causing inflammation and damage that triggers clotting.
Those clots can have physiological effects. Danny Jonigk, a pathologist at Hanover Medical School in Germany, and his colleagues looked at tissue samples from people who died of COVID-19. They found micro-clots and saw that the capillaries had split, forming new branches to try to keep oxygen-rich blood flowing10. The downside was that the branching introduces turbulence into the flow that can give rise to fresh clots.
How common is long COVID? Why studies give different answers
Several other labs have found signs that, in some people, this tendency towards clotting persists months after the initial infection. James O’Donnell, a haematologist and clotting specialist at Trinity College Dublin, and his colleagues found11 that about 25% of people who are recovering from COVID-19 have signs of increased clotting that are “quite marked and unusual”, he says.
What is less clear is whether this abnormal clotting response is actually to blame for any of the symptoms of long COVID, “or is it just, you know, another unusual phenomenon associated with COVID?” O’Donnell says.
Alex Spyropoulos, a haematologist at the Feinstein Institutes for Medical Research in New York City, says the micro-clot hypothesis presents “a very elegant mechanism”. But he argues that much more work is needed to tie the lab markers to clinical symptoms. “What’s a little bit disturbing is that these authors and others make huge leaps of faith,” Spyropoulos says.
Jeffrey Weitz, a haematologist and clotting specialist at McMaster University in Hamilton, Canada, points out that the method Pretorius’s team is using to identify micro-clots “isn’t a standard technique at all”. He adds: “I’d like to see confirmation from other investigators.” Micro-clots are difficult to detect. Pathologists can spot them in tissue samples, but haematologists tend to look for markers of abnormal clotting rather than the clots themselves.
Other, larger studies of long COVID have failed to find signs of clotting. Michael Sneller, an infectious-disease specialist, and his colleagues at the NIH in Bethesda, Maryland, thoroughly examined 189 people who had been infected with SARS-CoV-2, some with lingering symptoms and some without, and 120 controls12. They did not specifically look for micro-clots. But if micro-clots had been clogging the capillaries, Sneller says, they should have seen some evidence — tissue damage in capillary-rich organs such as the lungs and kidneys, for example. Micro-clots might also damage red blood cells, leading to anaemia. But Sneller and his colleagues found no signs of this in any of the lab tests.
The four most urgent questions about long COVID
Kell and Pretorius argue that just because this study didn’t find any evidence of micro-clots doesn’t mean they aren’t there. One of the key issues with long COVID is that “every single test comes back within the normal ranges”, Pretorius says. “You have desperately ill patients with no diagnostic method.” She hopes that other researchers will read their papers and attempt to replicate their results. “Then we can have a discussion,” she says. The ultimate causal proof, she adds, would be people with long COVID feeling better after receiving anticoagulant therapies.
There is some limited evidence of this. In an early version of a preprint, posted in December 2021, Kell, Pretorius and other researchers, including physician Gert Jacobus Laubscher at Stellenbosch University, reported that 24 people who had long COVID and were treated with a combination of two antiplatelet therapies and an anticoagulant experienced some relief13. Participants reported that their main symptoms resolved and that they became less fatigued. They also had fewer micro-clots. Pretorius and Kell are working to gather more data before they try to formally publish these results. But other physicians are already using these medications to treat people with long COVID. Some are even offering a dialysis-like procedure that filters fibrinogen and other inflammatory molecules from the blood. To O’Donnell, such treatment feels premature. He accepts that some people with long COVID are prone to clots, but leaping from a single small study to treating a vast number of people is “just not going to wash in 2022 in my book”, he says. Sneller agrees. “Anticoagulating somebody is not a benign thing. You basically are interfering with the blood’s ability to clot,” he says, which could make even minor injuries life-threatening.
Kell says he’s tired of waiting for a consensus on how to treat long COVID. “These people are in terrible pain. They are desperately unwell,” he says. Altmann understands that frustration. He gets e-mails almost daily, asking: “Where are the drug trials? Why does it take so long?” But even in the midst of a pandemic, he argues, researchers have to follow the process. “I’m not rubbishing anybody’s data. I’m just saying we’re not there yet,” he says. “Let’s join up the dots and do this properly.”
References
- Kell, D. B., Laubscher, G. J. & Pretorius, E. Biochem. J. 479, 537–559 (2022).PubMed Article Google Scholar
- Pretorius, E., Briedenhann, S., Marx, J. & Franz, R. C. Ultrastruct. Pathol. 30, 167–176 (2006).PubMed Article Google Scholar
- Pretorius, E. et al. Cardiovasc. Diabetol. 20, 172 (2021).PubMed Article Google Scholar
- Pretorius, E. et al. Cardiovasc. Diabetol. 21, 148 (2022).PubMed Article Google Scholar
- Nunes, J. M., Kruger, A., Proal, A., Kell, D. B. & Pretorius, E. Pharmaceuticals 15, 931 (2022).Article Google Scholar
- Grobbelaar, L. M. et al. Biosci. Rep. 41, BSR20210611 (2021).PubMed Article Google Scholar
- Swank, Z., Senussi, Y., Alter, G. & Walt, D. R. Preprint at medRxiv https://doi.org/10.1101/2022.06.14.22276401 (2022).
- Nyström, S. & Hammarström, P. J. Am. Chem. Soc. 144, 8945–8950 (2022).PubMed Article Google Scholar
- Ryu, J. K. et al. Preprint at bioRxiv https://doi.org/10.1101/2021.10.12.464152 (2021).
- Ackerman, M. et al. N. Engl. J. Med. 383, 120–128 (2020).PubMed Article Google Scholar
- Townsend, L. et al. J. Thromb. Haemost. 19, 1064–1070 (2021).PubMed Article Google Scholar
- Sneller, M. C. et al. Ann. Intern. Med. 175, 969–979 (2022).PubMed Article Google Scholar
- Pretorius, E. et al. Preprint at Research Square https://doi.org/10.21203/rs.3.rs-1205453/v1 (2021)