CDC officials were worried about causing panic.
By Zachary Stieber, 1/25/2024 The Epoch Times
The nation’s top public health agency did not send an alert on COVID-19 vaccines and heart inflammation because officials were concerned they would cause panic, according to an email obtained by The Epoch Times.
The U.S. Centers for Disease Control and Prevention (CDC) in 2021 drafted an alert for heart inflammation, or myocarditis, and the Pfizer-BioNTech and Moderna COVID-19 vaccines. Officials prepared to release it to the public, taking steps including having the agency’s director review the language, internal documents show.
The alert would have been sent through the CDC’s Health Alert System (HAN) network, which goes to state and local officials, as well as doctors, across the country.
The alert was never sent.
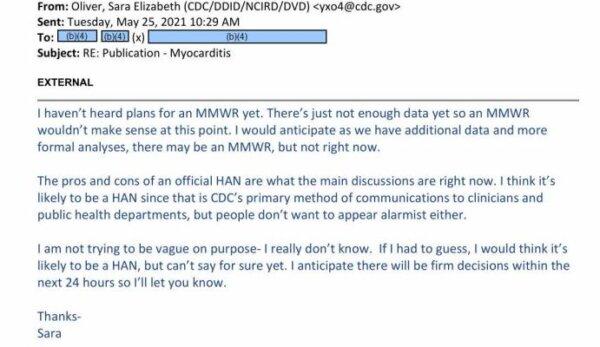
In the May 25, 2021, email, exclusively obtained by The Epoch Times, a CDC official revealed why some officials were against sending the alert.
“The pros and cons of an official HAN are what the main discussion are right now,” Dr. Sara Oliver, the official, wrote in the missive. “I think it’s likely to be a HAN since that is CDC’s primary method of communications to clinicians and public health departments, but people don’t want to appear alarmist either.
Dr. Oliver was corresponding with an employee of either Pfizer or Moderna. The employee’s name and email were redacted in the copy obtained by The Epoch Times.
Dr. Oliver did not respond to a request for comment. Asked about the email, the CDC did not address Dr. Oliver’s statement.
The “CDC’s apparent decision to not immediately issue a formal alert to clinicians warning them about the increased risk of myocarditis and pericarditis in vaccinated individuals is not only inexcusable, it’s malpractice,” Sen. Ron Johnson (R-Wis.), the top Republican on the Senate Homeland Security and Governmental Affairs Committee’s Permanent Subcommittee on Investigations, told The Epoch Times in an email.
“CDC should never prioritize its own public perception over the public’s health, and those who made the decision to do so must be held fully accountable,” he added.
It remains unclear which official or officials decided not to send the alert at a time when doctors across the country were seeing patients with myocarditis report to emergency rooms with chest pain and other symptoms.
Kim Witczak, a drug safety advocate who helped convince regulators to add a suicide warning to antidepressants, said the CDC’s move to downplay heart inflammation fits into a longstanding pattern of transparency issues with agencies and drug companies.
“I can’t even believe that this was even a discussion where they’re like, ‘We don’t want to alarm them.’ We do need to alarm people. We need people to be aware that this is a real potential [problem] that could happen,” Ms. Witzcak told The Epoch Times.
Those kinds of choices have helped erode consumer confidence in public health, she added.
Dr. Tom Frieden, a former CDC director who now serves as president and CEO of the global health project Resolve to Save Lives, also reviewed the messages.
“It is important to carefully weigh the risk of COVID-19 against the risk and benefit of any treatment, including the vaccine. The vaccine safety systems worked—they found a very rare but real signal of myocarditis soon after distributing vaccines that were administered to adolescents,“ Dr. Frieden told The Epoch Times via email. ”When public health officials see a safety signal, they must investigate whether it is ‘true’ or ‘random.’ It is important to consider multiple data angles and gather evidence from partners on the ground, including clinicians. This needs to be done quickly but carefully and thoroughly.”
Moderna, Pfizer Given Heads Up
U.S. authorities identified myocarditis and a related condition, pericarditis, before the vaccines were cleared as events that could be caused by the vaccines. People who received the Moderna and Pfizer vaccines began reporting myocarditis and pericarditis to health authorities and the vaccine manufacturers shortly after the vaccines were rolled out in December 2020.
A signal in the Vaccine Adverse Event Reporting System (VAERS), which the CDC helps manage, triggered in February 2021, the same month Israel warned the CDC and U.S. drug regulators of a “large number” of cases, primarily among young males.
Dr. Rochelle Walensky, the CDC’s director at the time, first addressed the issue publicly in April 2021. She falsely said the agency had seen no reports and that no signal had triggered, while disclosing the CDC was in touch with U.S. military officials on cases among service members.
In reality, hundreds of cases had been reported to the CDC, including some that resulted in death; the CDC either missed or ignored the signal in VAERS; and the CDC helped hide a signal that emerged from a Department of Veterans Affairs system, internal documents and other data reviewed by The Epoch Times show.
The CDC did communicate to certain state officials about myocarditis issues starting in April 2021 and told some doctors in a May 14, 2021, email that the agency was monitoring reports of the inflammation following Pfizer and Moderna vaccination.
Shortly after that missive was sent, the CDC began considering next steps, according to the newly obtained documents.
Dr. Oliver on May 21, 2021, emailed representatives of Moderna and Pfizer to warn them that the CDC was planning to go public with information on the myocarditis cases.
“Wanted to make sure you were aware before anything was made public,” Dr. Oliver wrote in one of the messages, which were obtained by The Epoch Times and are being reported in this story for the first time. “You may be aware, but there have been concerns for myocarditis seen in adolescents and young adults after receipt of the mRNA vaccines. Thankfully, the cases appear relatively mild, but there is concern that we need to make providers aware of this issue. CDC is discussing communication options, and we may have more information tomorrow.”
Cardiologists say there’s no such thing as a mild case of heart inflammation and research has since shown that a number of cases don’t resolve for months, if at all.
The Moderna and Pfizer vaccines both use modified messenger RNA (mRNA) technology.
Moderna and Pfizer did not respond to requests for comment.
One representative from Pfizer sent information to Dr. Oliver and colleagues ahead of a planned meeting, the emails show. The information was redacted.
Moderna officials met with the CDC on May 22, 2021. The discussion covered how the CDC was considering saying there was a “possible causal relationship,” or that the vaccines might be causing the inflammation, according to the emails.
Moderna asked how government officials thought the myocarditis was being caused, or the mechanism of action.
“My current understanding is that it isn’t necessarily a defined mechanism, but that we’ve seen very similar/consistent findings where mRNA vaccines have been used all occurring within days of receipt of an mRNA vaccine (although it could be that systemic inflammation plays a role),” Dr. Oliver wrote.
A representative with one of the companies then checked in on May 25, 2021, asking if the CDC had decided how to communicate to the public about myocarditis.
“Apologies that there hasn’t been more solid communication on this. Unfortunately, I still don’t have a firm update to share. Things have been changing rapidly here,” Dr. Oliver wrote. In the next email, she wrote that some officials did not want to cause panic.
“I am not trying to be vague on purpose- I really don’t know,” she said. “If I had to guess, I would think it’s likely to be a HAN, but can’t say for sure yet. I anticipate there will be firm decisions within the next 24 hours so I’ll let you know.”
Scaled-Down Response
A two-page draft of the alert obtained by The Epoch Times was completely redacted. The Epoch Times is working on acquiring an unredacted copy.
The draft was circulated internally, including to Dr. Walensky, emails show. The messages indicated the CDC chose not to send the alert after consulting with the U.S. Food and Drug Administration (FDA).
The CDC said on its website on May 20, 2021, that a review of post-vaccination myocarditis found “relatively few reports” and that rates of myocarditis “have not differed from expected baseline rates.”
Instead of the alert, the CDC decided to post another webpage called clinical considerations. The page, posted on May 27, 2021, said that “increased cases of myocarditis and pericarditis have been reported in the United States after mRNA COVID-19 vaccination (Pfizer-BioNTech and Moderna)” since April 2021.
The page also said the CDC and the agency’s partners were investigating the issue before recommending COVID-19 vaccination for everyone aged 12 and older.
A draft of the page was shared with Moderna and Pfizer at least several hours before publication, according to the emails.
A CDC spokeswoman said that safety data prompted the CDC to post information on myocarditis online “for public awareness and to provide guidance to clinicians.” She said the clinical considerations reached the same 300,000 provider recipients a HAN alert would have.
“A clinical consideration is useful when information needs to be updated as circumstances evolve, and more data is collected and evaluated,” the spokeswoman said.
In a separate email, she said that “CDC’s focus and concern on myocarditis after COVID-19 vaccination is well known and documented.”
An FDA spokesperson declined to detail its influence on the shelved alert.
“The FDA continues to work collaboratively with the CDC to monitor for known safety risks related to vaccines and determine how best to ensure any relevant safety information is conveyed to the public, health care providers and clinicians,” the spokesperson told The Epoch Times in an email. “After thorough assessment and when the potential risk was clear, the FDA updated the fact sheets for the COVID-19 vaccines and communicated with the public in a manner that was determined to be appropriate for the assessed risk.”
Federal rules require the FDA to add a warning about a “a clinically significant hazard as soon as there is reasonable evidence of a causal association with a drug; a causal relationship need not have been definitely established.”
The FDA on June 25, 2021, added warnings about myocarditis to the labels for the Pfizer and Moderna vaccines.