Authors: CDC
Summary of recent changes (last updated March 3, 2021)
- Considerations broadened to include use of Janssen (Johnson & Johnson) COVID-19 vaccine.
Key Points
Under the Emergency Use Authorizationsexternal icon for COVID-19 vaccines, appropriate medical treatment for severe allergic reactions must be immediately available in the event that an acute anaphylactic reaction occurs following administration of a COVID-19 vaccine. These interim considerations provide information on preparing for the initial assessment and management of anaphylaxis following COVID-19 vaccination.
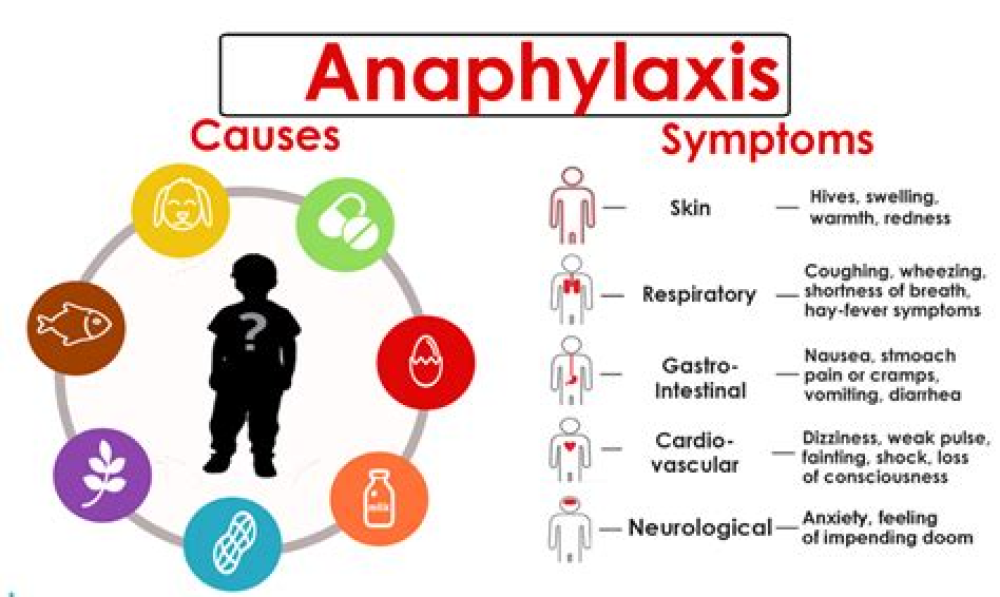
Overview
Anaphylaxis, an acute and potentially life-threatening allergic reaction, has been reported rarely following COVID-19 vaccination. These interim considerations provide recommendations on assessment and management of anaphylaxis following COVID-19 vaccination. Detailed information on CDC recommendations for vaccination, including contraindications and precautions to vaccination, can be found in the Clinical Considerations for Use of COVID-19 Vaccines Currently Authorized in the United States. Patients should be screened prior to receipt of each vaccine dose, and those with a contraindication should not be vaccinated. A COVID-19 prevaccination questionnaire pdf icon[6 pages] is available to assist with screening.
For More Information: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/managing-anaphylaxis.html