Authors: JACC State-of-the-Art ReviewAzita H. Talasaz, PharmD,a,bParham Sadeghipour, MD,cHessam Kakavand, PharmD,a,bMaryam Aghakouchakzadeh, PharmD,aElaheh Kordzadeh-Kermani, PharmD,aBenjamin W. Van Tassell, PharmD,d,eAzin Gheymati, PharmD,aHamid Ariannejad, MD,bSeyed Hossein Hosseini, PharmD,aSepehr Jamalkhani,cMichelle Sholzberg, MDCM, MSc,f,gManuel Monreal, MD, PhD,hDavid Jimenez, MD, PhD,iGregory Piazza, MD, MS,jSahil A. Parikh, MD,k,lAjay J. Kirtane, MD, SM,k,lJohn W. Eikelboom, MBBS,mJean M. Connors, MD,nBeverley J. Hunt, MD,oStavros V. Konstantinides, MD, PhD,p,qMary Cushman, MD, MSc,r,sJeffrey I. Weitz, MD,t,uGregg W. Stone, MD,k,vHarlan M. Krumholz, MD, SM,w,x,yGregory Y.H. Lip, MD,z,aaSamuel Z. Goldhaber, MD,j and Behnood Bikdeli, MD, MSj,k,w,∗
Abstract
Endothelial injury and microvascular/macrovascular thrombosis are common pathophysiological features of coronavirus disease-2019 (COVID-19). However, the optimal thromboprophylactic regimens remain unknown across the spectrum of illness severity of COVID-19. A variety of antithrombotic agents, doses, and durations of therapy are being assessed in ongoing randomized controlled trials (RCTs) that focus on outpatients, hospitalized patients in medical wards, and patients critically ill with COVID-19. This paper provides a perspective of the ongoing or completed RCTs related to antithrombotic strategies used in COVID-19, the opportunities and challenges for the clinical trial enterprise, and areas of existing knowledge, as well as data gaps that may motivate the design of future RCTs.
Thromboembolism in Patients With Coronavirus Disease-2019
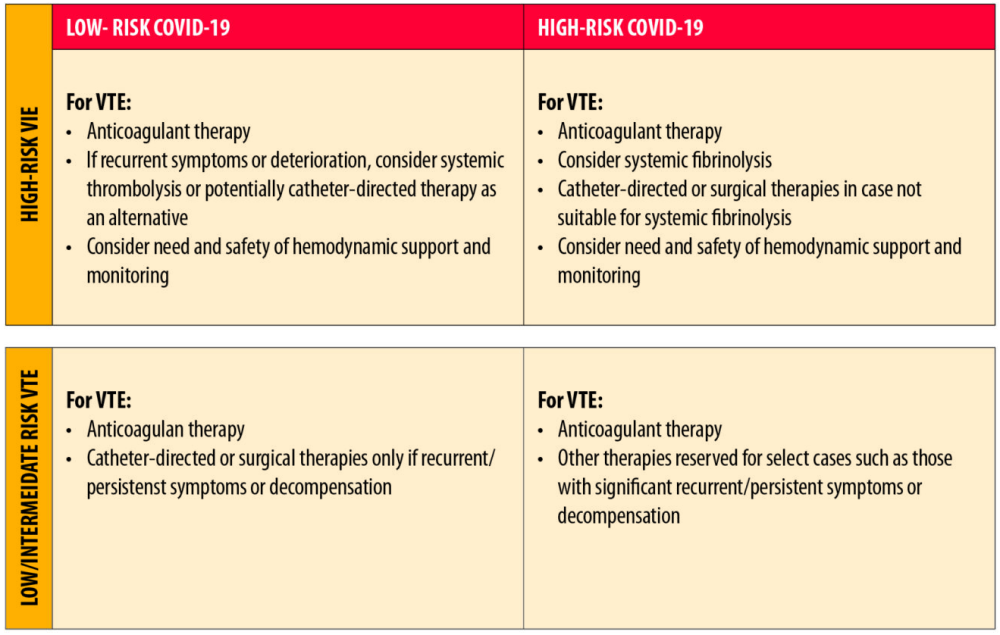
Microvascular and macrovascular thrombotic complications, including arterial and especially venous thromboembolism (VTE), seem to be common clinical manifestations of coronavirus disease-2019 (COVID-19), particularly among hospitalized and critically ill patients (1, 2, 3, 4). Pooled analyses have helped in providing aggregate estimates of thrombotic events (4,5). In a recent systematic review and meta-analysis, the overall incidence of VTE among inpatients with COVID-19 was estimated at 17% (95% confidence interval [CI]: 13.4 to 20.9), with variation based on study design and method of ascertainment; there was a four-fold higher incidence rate in patients in the intensive care units (ICUs) compared with non-ICU settings (28% vs. 7%) (6). In addition, postmortem studies show frequent evidence of microvascular thrombosis in patients with COVID-19 (7,8). The influence of these events on mortality rates remains unknown (9).Go to:
Pathophysiology of Thromboembolism in COVID-19: Virchow’s Triad in Action
COVID-19 can potentiate all 3 components of Virchow’s triad and increases the risk of thrombosis (Figure 1 ). First, severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) infection may trigger endothelial dysfunction. Using the angiotensin-converting enzyme 2, which is expressed on the surface of many cells, SARS-CoV-2 enters endothelial cells and may impair their intrinsic antithrombotic properties. It is proposed that viremia, hypoxia, the inflammatory response, increased expression of tissue factor, and elevated levels of neutrophil extracellular traps (NETs) can together disrupt the hemostasis equilibrium and promote endothelial activation (10, 11, 12). This induction of a procoagulant state along with the reduction in plasminogen activators further results in increased platelet reactivity (13, 14, 15). Inflammatory cytokines and endothelial activation can lead to downregulation of antithrombin and protein C expression. They can also lead to an increase in the levels of plasminogen activator inhibitor; fibrinogen; factors V, VII, VIII, and X; and von Willebrand factor (16). Increased platelet reactivity, NETosis, and alterations in the aforementioned hemostatic factors result in a hypercoagulable state (17, 18, 19, 20, 21, 22).
For More Information: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7963001/