SEPTEMBER 12, 2023 by Maria Fernanda Ziegler, FAPESP Medical Express
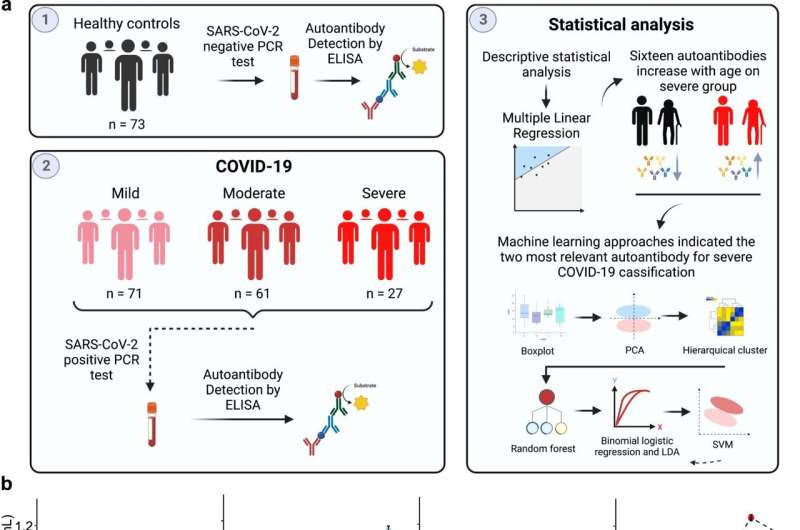
An article published in npj Aging reveals that natural production of auto-antibodies increases with age, and that infection by SARS-CoV-2 can exacerbate production of auto-antibodies relating to auto-immune diseases, helping to explain why aging increases the chances of developing severe COVID-19. The study also discovered some of the factors that associate the severe form of the disease with blood clotting disorders such as thrombosis.
“These findings open the door to a better understanding of many pathological events associated with COVID-19, ranging from memory loss to sudden death from acute thrombosis. The hyperinflammation typical of severe COVID-19 and the increase in auto-antibodies produce blood clotting, resulting in the need to reconstruct tissue and recruit a coagulation cascade. Thrombosis and inflammation are closely correlated. Our study is one more confirmation of this important link,” Otávio Cabral-Marques, a professor at the University of São Paulo’s Medical School (FM-USP) in Brazil and last author of the article, told Agência FAPESP.
Researchers in Germany and the United States collaborated on the study.
Another interesting aspect of the study, according to Cabral-Marques, is that the rise in levels of auto-antibodies—antibodies (immune proteins) that may harmfully react with a person’s own tissues or organs—in severe COVID-19 cases could also be true of other pathologies.
“It’s not exclusive to SARS-CoV-2. Other pathogens also raise levels of auto-antibodies, so that they can serve as markers or therapeutic targets to save the lives of patients with almost terminal sepsis, for example,” Cabral-Marques said.
The study involved an analysis of auto-antibody data for 159 COVID-19 patients (71 mild, 61 moderate, and 27 severe) and 72 healthy individuals as the control group. “When we cross-referenced the data using artificial intelligence techniques, we discovered two main auto-antibodies that were exacerbated in patients with severe symptoms. They were precisely the auto-antibodies associated with blood clotting and thrombocytopenia [low blood platelet count, heightening the risk of hemorrhage],” said Dennyson Leandro M. Fonseca, first author of the article.
High levels of these auto-antibodies, he explained, can lead the organism to harm itself by producing blood clots and netosis—an immune system mechanism involving regulated cell death via the formation of neutrophil extracellular traps (NETs), networks of DNA that bind to pathogenic microbes. In certain cases, NETs serve as markers of alveolar inflammation and may contribute to tissue injury.
“When we divided the data into younger and older patients, we found that although this mechanism may occur in under-50s, it makes the elderly more susceptible to severe COVID-19. This explains why old age is one of the main risk factors for COVID-19,” he said.
As noted, a mild increase in production of auto-antibodies is part of the natural aging process, but the researchers observed that infection by SARS-CoV-2 exacerbates levels of auto-antibodies in severe patients.
Previous studies showed that auto-antibodies targeting proteins in the first line of anti-viral defense (interferons) are present in the general population and increase dramatically in older COVID-19 patients. The study reported in npj Aging extends the classes of auto-antibodies associated with aging and confirms that their impact varies according to age and COVID-19 severity.
The group discovered that severe COVID-19 patients display a significant age-related increase in auto-antibodies against 16 targets, including amyloid β peptide, β catenin, cardiolipin, claudin, enteric nerve, fibulin, insulin receptor A and platelet glycoprotein.
“This discovery provides new avenues for validation of the action mechanism of auto-antibodies and broadens the pool of targets, providing a more comprehensive picture of the underlying pathophysiology of COVID-19 disease progression with the advance of aging and permitting more suitable interventions,” Cabral-Marques said.
Systemic problem
Severe COVID-19 is known to cause profound organ damage due to a combination of auto-inflammatory and auto-immune responses. This process can result in myopathy (muscle disorders), vasculitis (blood vessel inflammation), arthritis (joint inflammation), anti-phospholipid syndrome (APS) associated with deep vein thrombosis, pulmonary embolism and stroke, as well as other damage to lungs, kidneys and the neurological system.
Immunological dysregulation is also frequent in post-COVID syndrome, causing heterogeneous symptoms such as fatigue, vascular dysfunction, pain, neurological manifestations and neuropsychiatric problems.
The most well-known inflammatory response associated with COVID-19 is the “cytokine storm,” which basically consists of heightened release of cytokines (the molecules responsible for alerting the immune system to the need to send more defense cells to the infection site), leading to exacerbated inflammation and dysregulation of other signaling systems. Another is hyperactivation of macrophages, immune system cells that promote auto-antibody production.
Which comes first?
The authors of the article are not yet sure which comes first—the severity of COVID-19 or the rise in levels of auto-antibodies.
“We analyzed these two directions statistically and believe they’re both mutual and bidirectional,” Fonseca said. “Auto-antibodies may interact, in conjunction with other immune system molecules, in a highly complex network that underlies immunopathological processes in severe COVID-19, or they may be boosted by health problems associated with aging and make the disease progress to a severe condition.”
Severe COVID-19, he noted, promotes an environment in the organism involving tissue injury (acute respiratory distress syndrome), cytokine storm and macrophage hyperactivation, all of which fosters the production of auto-antibodies. In turn, this context could allow auto-antibodies to act synergistically with multiple metabolites, cytokines and chemokines, which are naturally dysregulated in elderly patients as part of immunosenescence, resulting in increased susceptibility to infections and auto-immune disorders.
