
VAERS Summary for COVID-19 Vaccines through 2/4/2022: Adverse Events
https://vaersanalysis.info/2022/02/11/vaers-summary-for-covid-19-vaccines-through-2-4-2022/[...]
Read More
https://vaersanalysis.info/2022/02/11/vaers-summary-for-covid-19-vaccines-through-2-4-2022/[...]
Read More
https://vaersanalysis.info/2022/02/04/vaers-summary-for-covid-19-vaccines-through-01-28-2022/[...]
Read More
Official Reported Vaccine Adverse Events in the FDA Data Base https://vaersanalysis.info/2022/01/28/vaers-summary-for-covid-19-vaccines-through-01-21-2022/[...]
Read More
Three military doctors say medical billing code data captured by Defense Medical Epidemiology Database shows sharp spikes in miscarriages, myocarditis, cancer diagnoses, Bell's palsy, female infertility. According to the data…[...]
Read More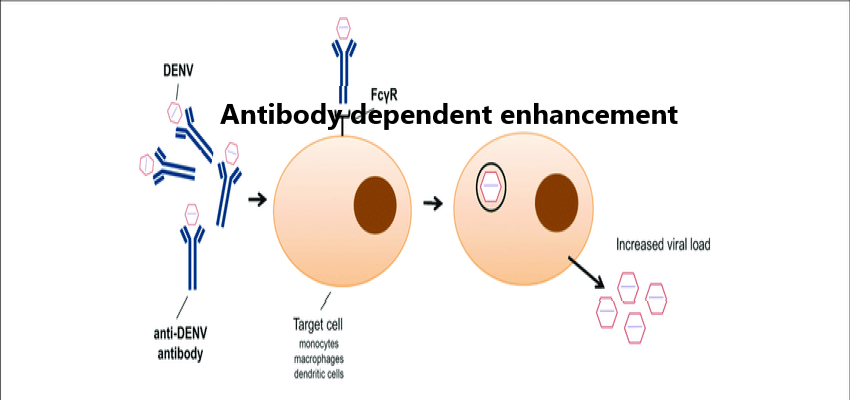
Authors: Lele Xu, Zhiqian Ma, Yang Li, Zhaoxia Pang, and Shuqi Xiao* Adv Immunol. 2021; 151: 99–133.Published online 2021 Sep 4. doi: 10.1016/bs.ai.2021.08.003 PMCID: PMC8438590PMID: 34656289 Abstract In some cases, antibodies can enhance virus entry and replication in cells.…[...]
Read More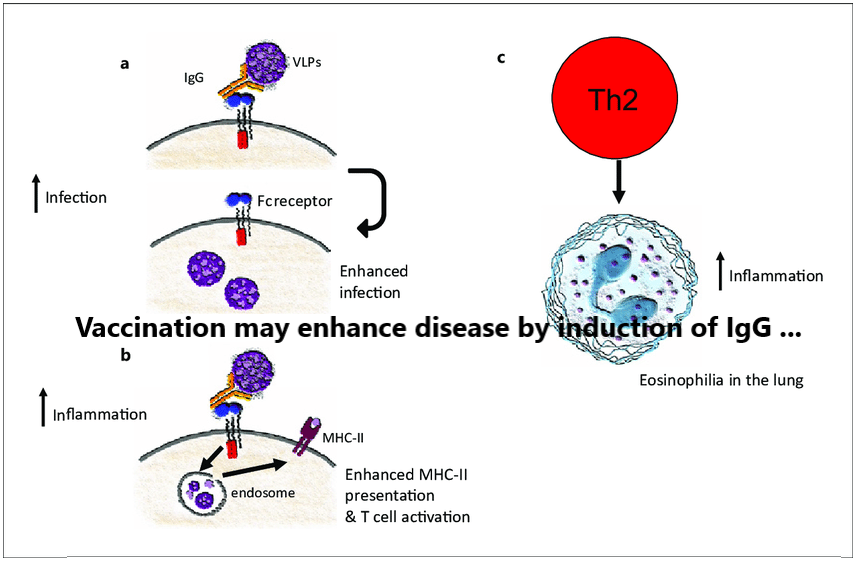
Authors: Flor M. Munoz,a,⁎Jakob P. Cramer,bCornelia L. Dekker,cMatthew Z. Dudley,dBarney S. Graham,eMarc Gurwith,fBarbara Law,gStanley Perlman,hFernando P. Polack,iJonathan M. Spergel,jEva Van Braeckel,kBrian J. Ward,lArnaud M. Didierlaurent,mPaul Henri Lambert,m and for the Brighton…[...]
Read More
Latest UK Data Shows Covid Infection RATE Among the Triple Jabbed (Boosted) Is HIGHER And RISING FASTER Than The Unvaccinated Across ALMOST EVERY Age Group 20 January 2022 Executive summaryFour…[...]
Read More
Official Reported Vaccine Adverse Events in the FDA Data Base https://vaersanalysis.info/2022/01/14/vaers-summary-for-covid-19-vaccines-through-01-07-2022/[...]
Read More
Official Reported Vaccine Adverse Events in the FDA Data Base For Detailed Graphs, Charts & Data https://vaersanalysis.info/2022/01/07/vaers-summary-for-covid-19-vaccines-through-12-31-2021/[...]
Read More
Official Reported Vaccine Adverse Events in the FDA Data Base For Detailed Graphs, Charts & Data https://vaersanalysis.info/2021/12/31/vaers-summary-for-covid-19-vaccines-through-12-24-2021/[...]
Read More