Authors: Lele Xu, Zhiqian Ma, Yang Li, Zhaoxia Pang, and Shuqi Xiao*
Adv Immunol. 2021; 151: 99–133.Published online 2021 Sep 4. doi: 10.1016/bs.ai.2021.08.003 PMCID: PMC8438590PMID: 34656289
Abstract
In some cases, antibodies can enhance virus entry and replication in cells. This phenomenon is called antibody-dependent infection enhancement (ADE). ADE not only promotes the virus to be recognized by the target cell and enters the target cell, but also affects the signal transmission in the target cell. Early formalin-inactivated virus vaccines such as aluminum adjuvants (RSV and measles) have been shown to induce ADE. Although there is no direct evidence that there is ADE in COVID-19, this potential risk is a huge challenge for prevention and vaccine development. This article focuses on the virus-induced ADE phenomenon and its molecular mechanism. It also summarizes various attempts in vaccine research and development to eliminate the ADE phenomenon, and proposes to avoid ADE in vaccine development from the perspective of antigens and adjuvants.
Keywords: Coronavirus, Antibody-dependent infection enhancement, Vaccine.
1. Introduction
In 1967 Hawkes first confirmed that IgG in serum can induce ADE (Lafferty, 1967). Many viruses have been proven to have ADE effects, such as Arthropod borne viruses (ABV), Dengue virus (DENV), Respiratory syncytial virus (RSV), Human Immunodeficiency virus (HIV), Feline infectious perionitis virus (FIPV), Coronaviruses (CoV). For many viruses (including DENV, HIV) that pose a major threat to human health, the presence of ADE is considered to be a major obstacle to vaccine development.
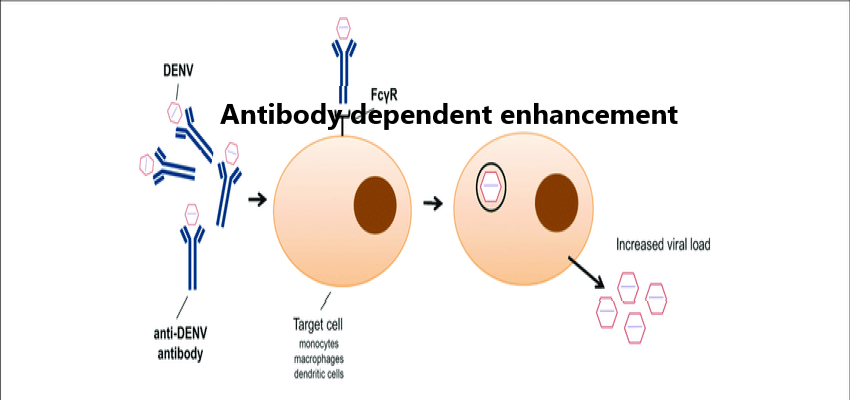
Fc receptor (FcR)-mediated ADE is the most common form of ADE, which was first discovered by Halstead (1977). At first, it was thought that the antigen antibody immune complex formed by virus and specific antibody combined with the host cell with the help of FcR on the cell surface, which was more conducive to the entry of virus, and increased the infection amount and infection rate of virus, finally led to the increase of infection and replication of virus. By phagocytosing immune complexes, the cells expressing FcR on the surface, such as monocytes, macrophages, dendritic cells, and certain granulocytes, can produce ADE. This kind of ADE is mainly mediated by IgG antibody, but IgM, IgE and IgA antibody have also shown the ability of ADE (Janoff, Wahl, Kelly, & Smith, 1995; Shi et al., 2018; Takada, Ebihara, Feldmann, Geisbert, & Kawaoka, 2007).
Complement mediated ADE(C-ADE) refers to the combination of virus and antibody to form an immune complex, which activates the complement and combines with the complement to form a complex, and then enters the cell through the complement receptor on the cell surface. After the complement inactivation, the ability of the serum to mediate the enhancement of viral infection decreased. However, the enhanced effect of virus infection returned to normal after the addition of excessive complement which indicated that C-ADE needed the participation of complement and antibody. The antibody of CR3 can block ADE infection of West Nile virus on cells expressing FcR, while the antibody of FcR receptor cannot block ADE infection, indicating that ADE infection can also be mediated by complement alone (Gordon, 1983).
With the research on ADE, the concept of intrinsic antibody-dependent enhancement(iADE) was proposed (Chareonsirisuthigul, Kalayanarooj, & Ubol, 2007; Halstead, Mahalingam, Marovich, Ubol, & Mosser, 2010). Combining Fc and FcR also changes intracellular signaling pathways, causing them to shift from antiviral mode to viral promotion mode. This process is called iADE. In other words, ADE changes the innate immune response in cells and inhibits the antiviral response in cells, thus enhancing the virus infection. iADE infection can change some molecular signal transduction pathways in the process of immune cell response, especially the changes in the expression of IFN-β and IL-10, the changes in the phosphorylation levels of key molecules in signal transduction (NF-κB, STAT and IRF, etc.) (Patro et al., 2019; Taylor et al., 2015; Tsai et al., 2014; Ubol, Phuklia, & Kalayanarooj, 2010). iADE could be the key to tackling the dangers of ADE in the future.
The mechanism or interaction of ADE is not fully understood. It is necessary to understand the upstream and downstream molecular signal events of ADE. In the development of vaccines for a variety of viral diseases, ADE needs to be overcome.
2. ADE of representative virus
2.1. Coronavirus
The mechanism of MERS ADE was mediated by neutralizing MAb targeting the coronavirus S protein RBD (Wan et al., 2020). ADE of MERS-CoV followed the same entry pathway as that of DPP4-dependent MERS-CoV. RBD specific neutralizing monoclonal antibody mediates coronavirus entry into ADE by functionally mimicking viral receptors. If other parts of the targeted neutralizing antibody do not trigger the conformational change of the spike, they are unlikely to mediate ADE. Similar findings have been found in SARS-CoV. The monoclonal antibody specific to SARS-CoV RBD (S230) binds to ACE2 in SARS-CoV RBD, triggering the conformational change of SARS-CoV. S230 can block the connection with DPP4 or ACE2 through the competitive mechanism, respectively. The antibody attributes membrane fusion by mimicking the action of the receptor (Walls et al., 2019). Therefore, SARS-CoV is likely to have ADE mechanism similar to MERS-CoV.
In the acidic pH environment of the endosome leads to the aggravation of antibody mediated infection, which is the opposite of SARS-CoV infection mediated by ACE2 receptor. Virus particles that infect cells via ADE pathway may still be trapped in acid compartment, and inhibition of internal acidification will prevent its degradation. It was also found that the sera induced by ADE did not contain anti-Spike IgG2a antibody, while the two neutralized/non-enhanced sera did (Jaume et al., 2011). IgG2a is a marker of Th1 type response, and Th2 type is more prone to induce ADE. Whether this phenomenon is related to the preference for Th1 type response and whether the deficiency of IgG2a has a causal relationship with the occurrence of ADE infection needs further research.
Daniel found two VHH (variable domain of the heavy-chain of heavy chain antibody) targeting SARS-CoV and MERS-CoV RBD, which neutralizes SARS-CoV and MERS-CoV and interferes with receptor binding (Wrapp et al., 2020). The possible mechanisms of neutralization are the blocking of the receptor binding interface and the capture of the up-conformation of RBDs, acting as receptor simulators that trigger the premature transition from the prefusion-to-post fusion conformation. The author did not discuss the possibility of this antibody ADE, but according to Wan’s results mentioned above (Wan et al., 2020), it is likely to have the phenomenon of ADE. It should be noted that the VHH directed by SARS-CoV RBD reacts with SARS-CoV-2 RBD, and can block receptor binding. SARS-CoV-2 and SARS-CoV have 79.6% sequence identity (Zhou et al., 2020). They use the same receptor ACE2 and cause similar acute respiratory syndrome. There is a cross reaction between SARS-CoV-2 and SARS-CoV S protein antibody, but cross neutralization reaction is rare (Lv et al., 2020).The key epitope of ADE induced by SARS-CoV S protein has been identified (LYQDANC), which is highly similar in SARS-CoV-2 region (Wang & Zand, 2020; Wang et al., 2016). 48 kinds of SARS-CoV-2 antibodies were isolated by Zhou, 11 (23%) of which significantly increased the level of SARS-CoV-2 ADE in vitro, and 9 of them were RBD binding antibodies. It was further confirmed that the epitope of RBD was related to SARS-CoV-2 ADE in vitro (Zhou et al., 2021). Recent studies have found that antibodies that can promote SARS-CoV-2 infection can bind to specific sites of N-terminal domain(NTD), which leads to the conformational change of RBD and makes it easier to bind to ACE2 (Liu et al., 2021).
Observations in patients with SARS-CoV-2 were similar to those with SARS during the 2003 epidemic (Cheung et al., 2005; Tetro, 2020). It is speculated whether individuals with severe disease may have been exposed to one or more coronavirons and are experiencing an ADE due to antigen epitope heterogeneity (Tetro, 2020). ADE has been found in SARS-CoV, and is believed to be one of the reasons for such a high mortality rate (Ho et al., 2005). High concentration of anti SARS-CoV antiserum neutralized the infection of SARS-CoV, while highly diluted antiserum significantly increased the infection of SARS-CoV and induced a higher level of apoptosis (Wang et al., 2014). Rhesus monkeys immunized with S-glycoprotein of full-length SARS-CoV can cause serious acute lung injury (ALI) when they attack the virus (Chen et al., 2005; Liu et al., 2019). All these suggest that the development of SARS-COV vaccine requires special attention to ADE. The SARS-CoV S protein trimer-immunized hamster serum mediated the ADE of SARS-CoV, but the viral load and lung lesions did not increase in the vaccinated animals. These data indicate that the enhancement of virus entry into cells in vitro cannot fully predict the adverse effects in vivo (Arvin, Fink, Schmid, Cathcart, & Virgin, 2020; Jaume et al., 2011). Therefore, more in-depth research is needed to correlate in vitro laboratory test results with clinical results, so as to provide a reference for the SARS-CoV-2 ADE study.
At present, the clinical experience is not enough to prove the existence or non-existent of ADE in SARS-CoV-2 (Arvin et al., 2020). Without the support of data to believe that ADE will definitely hinder the development of SARS-CoV-2 vaccine. However, the experience of dengue fever vaccine and RSV vaccine reminds us that if there is a risk of ADE in the COVID-19 vaccine, special attention should be paid to the safety of any candidate SARS-CoV-2 vaccine (Coish & MacNeil, 2020; Sharma, 2020). The good news is that immunity to SARS-CoV-2 RBD or inactivated SARS-CoV-2 vaccine can cause a strong neutralizing antibody response in rodents, and antiserum does not mediate ADE (Gao et al., 2020; Quinlan et al., 2020). Even though some antibodies can enhance virus infection in cell experiments, they have not been shown to enhance virus infection in vitro experiments by mice and monkeys (Li, Edwards, Manne, Martinez, & Saunders, 2021).
2.2. Flaviviruses
Perhaps the most famous example of ADE infection is the virus-specific antibody enhanced DENV (Izmirly, Alturki, Alturki, Connors, & Haddad, 2020). The main representative viruses include Moray Valley encephalitis virus (MVEV), West Nile virus (WNV), Japanese encephalitis virus (JEV) and DENV(Schweitzer, Chapman, & Iwen, 2009; Weaver & Barrett, 2004).
Dengvaxia (CYD-TDV) is first licensed vaccine. This vaccine is only approved in dengue-endemic countries and only dengue seropositive people. CYD-TDV has shown a powerful effect in preventing serious diseases. But it increases the risk of severe dengue fever in seronegative patients. The potential risk of ADE is undoubtedly the difficulty of dengue fever vaccine development (Izmirly et al., 2020; Sridhar et al., 2018). DENV has four different serotypes (DENV1-4), ADE is easily caused between different serotypes (Heinz & Stiasny, 2012). Global epidemiological studies show that the vast majority of DHF/ DSS cases occur after the secondary infection of DENV. In addition, it has been proposed that ADE is also triggered by sub-neutralization concentrations DENV antibodies. From the perspective of mechanism, antibody mediated DENV entry into cells expressing FcR, such as monocytes, leads to increased viral replication, which triggers the release of inflammatory and vasoactive mediators, thus aggravating the severity of the disease. The critical time for DHF/DSS is approximately 2 months after degrade below a protective level of maternal dengue 2 neutralizing antibodies, maternally derived DENV-reactive IgG is a determinant of the viral burden in vivo (Chau et al., 2008, Chau et al., 2009; Kliks, Nimmanitya, Nisalak, & Burke, 1988). ADE can also be reproduced in vitro by primary human monocytes, macrophages, and mature DC, as well as cell lines expressing human and mouse Fc tissues (e.g., U937, K562, THP-1, and P388D1) in the presence of subneutralizing concentrations of antibodies. In addition, the expression of FcγRs can also promote ADE in non Fc cell lines (Goncalvez, Engle, Claire, Purcell, & Lai, 2007; Chareonsirisuthigul et al., 2007; Halstead, Chow, & Marchette, 1973; Littaua, Kurane, & Ennis, 1990; Morens, Larsen, & Halstead, 1987).
DENV could be divided into the compact and expanded mature DENV, the fully immature DENV, and the partially mature DENV. Some of these DENV particles are completely infectious, while others need to be infectious by binding with other molecules (Lok, 2016). The prM is a precursor protein of a membrane protein (M), which is present on the surface of immature DENV. The prM forms a heterodimer with the E protein, blocks the fusion peptide of the E protein, and prevents membrane fusion of the virus and the host cell membrane. In the process of virus maturation, prM is cleaved into M protein by furin protease under the induction of low pH, which rearranges E protein, and makes immature virus particles that are not infectious become mature virus and become infectious. Antibodies to prM mediate the binding of immature virus to target cells, thereby enhancing the infectivity of the virus. Using JEV Pr instead of DENV Pr will not change the neutralization ability of anti PrM antibody, and will not enhance the infection of K562 cells with Fc receptor, so as to avoid the ADE of anti prM antibody (Wang et al., 2017). Recent studies have found that the DENV gene for ADE is located at the fifth, sixth, seventh, and sixteenth amino acids of pr4, which can reduce the occurrence of ADE by replacing key amino acids (Cui et al., 2021).
Renner confirmed two neutralizing antibodies of DENV2, 2C8 can induce ADE, while 3H5 does not. Fc region of 3H5 (IgG1 subtype) was switched to Fc region of 2C8 (IgG2a subtype), it did not change this feature, which indicated that the difference was not caused by different IgG subtypes. 2C8: DENV can strongly bind to FcγR, but 3H5: DENV cannot bind to FcγR receptor. The binding of 3H5 antibody may lead to the deformation of virus surface, and the organization of the whole immunoglobulins will be too crowded to allow FcγR to bind. On the contrary, The upright conformation of 2c8 will completely expose the Fc region of the whole immunoglobulin, so that it can combine with FcγR to induce ADE (Morrone & Lok, 2019). 3H5 showed a strong binding force at neutral pH and low pH, while 2C8 showed a significant reduction in binding force at low pH. Due to the low pH value of the late endosomes, 2C8 may dissociate from the surface of the virus, leading to infection, which partly explains the mechanism of ADE in mature dengue granules (Lok, 2016; Morrone & Lok, 2019).
It should also be noted that DENV and ZIKV belong to the Flaviviridae genus in the virus classification, and there is a large amount of antigen overlap between them. DENV and ZIKV infection can induce cross-reactive antibody responses (Rathore & St John, 2020). The antibodies of one virus may promote the development of the other virus. Two immunodominant epitopes, the precursor membrane protein (prME) and the envelope (E) protein, can be recognized by cross-reactive antibodies. These antibodies not only neutralize the ills, but also promote viral replication and disease severity through Fc receptor-mediated myeloid cell infection. Serum from patients infected with DENV, as well as DENV-specific human monoclonal antibodies, bind to ZIKV and promote its infection of cells carrying Fc receptors. The broadly neutralizing anti-DENV E protein linear fusion ring epitope (FLE) are not neutralize to ZIKV, but can increase ZIKV infection (Dejnirattisai et al., 2016; Paul et al., 2016). Cross-reactive flavivirus antibodies may also cause other harmful effects of ZIKV infection by promoting placental transmission via FcRn-mediated endocytosis (Hermanns et al., 2018; Langerak et al., 2019). In the case of ADE, the co-infection of DENV and ZIKV will cause more damage to the host. The initial virus (ZIKV) will produce a higher viral load and a second peak, while the second virus (DENV) will produce a larger peak viral load and an earlier peak. ADE may have a greater impact on the second virus during co-infection (Tang et al., 2020). Antibodies produced against ZIKV or DENV could enhanced the entry of ROCV, SLEV, WNV and ILHV into K562 cells, which may lead to a risk of serious infection (Oliveira et al., 2019). The serum of WNV antibody-positive persons can significantly enhance ZIKV infection in vitro. Considering the prevalence of WNV infection in the United States, this may have a great potential risk. Further research found that the flavivirus E antibody is related to this cross ADE (Garg et al., 2021).
2.3. Porcine reproductive and respiratory syndrome virus (PRRSV)
PRRSV is an arteritis virus with a capsule (Karl-Klaus, Nico, et al., 1993). It is one of the most serious diseases faced by the global pig industry and causes huge losses to the pig industry every year (Li et al., 2021; Wang, Yu, Cai, Zhou, & Zimmerman, 2020).
ADE is present in PRRSV infection both in vivo and in vitro. In vitro, PRRSV infection was added with a certain concentration of PRRSV antibodies for 1 hour, and then the mixture was used to infect porcine alveolar macrophages, which was significantly higher than the control group by tens of times (Cancel-Tirado, Evans, & Yoon, 2004). In vivo studies have found that the mean level and duration of vireaemia in pigs treated with PRRSV-specific IgG subneutralization were greater than in control pigs injected with normal serum globulin (Yoon, Wu, Zimmerman, Hill, & Platt, 1996). This suggests that when maternal PRRSV-specific antibody, exposure to wild-type or vaccine PRRSV-induced antibody level decreases, ADE of PRRSV may occur, resulting in increased disease severity and possibly increased susceptibility of pigs to PRRSV infection. FcγR is the main way to mediate PRRSV ADE. FcγRI, FcγRIIb and FcγRIII are all related to ADE (Gu et al., 2015; Qiao et al., 2011; Shi et al., 2019). After infection with the PRRSV-antibody complex, the transcription level of FcγRI slightly increased, while the transcription level of FcγRIIb (inhibitor receptor) increased significantly. In addition to the receptor of IgG, the receptor FCɛRI of IgE also affects PRRSV infection. FcɛRI may be related to the antigen presenting process and regulation of the inflammatory response, PRRSV reproduction and the regulation of antiviral response during PRRSV infection (Shi et al., 2018).
Cancel-Tirado used a variety of monoclonal antibodies against M/N/GP3/CP5, and found that some monoclonal antibodies not only failed to inhibit virus infection, but also promoted virus replication (Cancel-Tirado et al., 2004). GP5 exists the main neutralizing epitope of the virus. There are two B-cell antigenic sites A and B on the GP5 protein. A epitope is the decoy epitope, and the antibody induced by A epitope has no neutralizing activity. Non-neutralizing antigen site A can be recognized by non-neutralizing antibody, and cannot be recognized by monoclonal antibody ISU25-c1 and pig neutralizing serum antibody. The A epitope is highly variable in the strains and has an immune advantage. Antibodies at this epitope appear early in PRRSV infection. Therefore, the epitope of GP5 antibody that can induce ADE is presumed to be epitope A. The antibody against N protein is the earliest and most common antibody after infection, but the N protein is not exposed on the virus surface. The antibody against N protein ISU15A can promote PRRSV infection. This process should not be an antibody against N that promotes virus adsorption and entry into cells. It is speculated that it may affect the replication of PRRSV in cells. Carmen a. Sautter’s study showed that immunized with the inactivated PRRSV vaccine and then attacked the homologous virulent virus, the vaccine-mediated enhancement of clinical symptoms was observed, but the results showed that it was not neutralizing antibody or other antibody mediated enhanced infection, increased the release of virus or the production of cytokines (Sautter, Trus, Nauwynck, & Summerfield, 2019). However, the heat-inactivated serum used in the test may affect the participation of complement, and the test is the use of monocyte Derived Macrophages. The immune complex enhanced infection may target other cells, such as FcR on monocytes and dendritic cells.
3. The molecular mechanism that produces ADE
3.1. FcγR-mediated ADE
FcγR, also known as IgG Fc receptors for immunoglobulin G, is an important cell surface molecule that is widely expressed on the surface of most immune cells and can specifically bind to the Fc region of immunoglobulin. At present, the identified human FcγR can be divided into three subclasses: FcγRI (CD64), FcγRI (CD32), FcγRIII (CD16). In addition, specific FcRIV was found in mice (Nimmerjahn, Bruhns, Horiuchi, & Ravetch, 2005; Taylor et al., 2015). As a bridge between humoral immunity and cellular immunity, FcγR mediates the interaction between immune complex and immune cells through specific binding with Fc region of immunoglobulin, so as to participate in the activation and regulation of various immune effects (de Taeye, Rispens, & Vidarsson, 2019). FcγR can also mediate the interaction between immune cells. The immune response mediated by FcγR includes the elimination of immune complex, the regulation of antibody production, the lysis of tumor cells, antigen processing and presentation, and the regulation of T cell proliferation and differentiation. FcγR plays a role in resisting the invasion of foreign microorganisms by triggering various immune functions of immune cells.
The connection between FcγR and viral immune complex can control ADE through internalization, and the result is the balance of activation and inhibition of FcγR. FcγRI, FcγRII and FcγRIII can all mediate the occurrence of ADE, but their action modes and intensity are different. In the cells expressing FcR, the production of cytokines mediated by FcγR depends on the proportion of activated and inhibited FcγR.
FcγRI is a high affinity receptor and the only FcR binding to IgG monomer. It consists of extracellular regions of three Ig like domains, a transmembrane domain and an intracellular domain (Hanson & Barb, 2015). It is mainly expressed on the surfaces of macrophages, monocytes, polymorphonuclear cells and dendritic cells. FcγRI is mainly involved in the process of antigen intake, processing and presentation in the early stage of immune response.
It has been proved that FcγRI can mediate the ADE of DENV, HIV, PRRSV and other viruses. The extracellular domain of FcγRI is sufficient to internalize the immune complex of infectious dengue virus through a mechanism that does not involve classical ITAM dependent signaling (Schlesinger & Chapman, 1999). Rodrigo found that enhanced immune complex infectivity mediated by FcγRIA was greatest when the receptor was associated with a γ-chain in its native form and that abrogation of γ-chain ITAM signaling capacity by Tyr-to-Phe mutation reduced but did not entirely eliminate this function (Rodrigo, Jin, Blackley, Rose, & Schlesinger, 2006). FcγRIA may have at least two internalization mechanisms of viral immune complexes. The first is γ-chain signal dependence. Aggregates of infectious viral immune complexes of sufficient size trigger the classical phagocytosis entry pathway. The second is a somewhat less efficient entry mechanism that did not require γ-chain activation and relied simply on concentrating partially neutralized virions onto the cell for entry by a parallel endocytosis mechanism (Rodrigo et al., 2006). The study found six different alternatively spliced of porcine FcγRI, and different FcγRI alternatively spliced have different effects on PRRSV ADE. The membrane pCD64-T1 enhances the replication of PRRSV in the form of promoting the endocrine of the PRRSV-antibody complex. Soluble pCD64-T3 exhibits inflammation-enhancing effects. It is speculated that pCD64-T3 can enhance the activation of TLR in ADE mode, and up-regulate the transcription of inflammatory factors such as TNF-α, IL-1β and IFN-β, and negatively regulate PRRSV ADE. Porcine FcγRI plays a dual regulatory role in PRRSV infection and PRRSV inflammation through alternatively spliced mechanism (Shi et al., 2019). Different spliceosomes lead to different viral infection results, which also increase the complexity of PRRSV ADE.
FcγRII is a low-affinity receptor and requires high-affinity binding through IgG immune complexes. Among them, FcγRIIA and FcγRIIC are activated receptors, while FcγRIIB is an inhibitory receptor (Guilliams, Bruhns, Saeys, Hammad, & Lambrecht, 2014). The activated receptor participates in intracellular signal transduction through immunoreceptor tyrosine-based activation motif (ITAM), for example, the combination of the antibody-virus complex of Ebola virus with the cell surface Fc eagerly RIIa will trigger the phosphorylation of Src family PTK and activate the subsequent signal transduction pathway, thereby leading to increased viral uptake through phagocytosis and/or macropinocytosis (Furuyama et al., 2016). The inhibited receptor contains the suppressed motif based on the immunoreceptor tyrosine-based inhibitory motif (ITIM) in its cytoplasmic domain. FcγRIIb mainly exerts its negative regulatory function through a series of cascade reactions generated after phosphorylation of its intracellular ITIM motif.
FcγRIIA is more effective than FcyRIA in enhancing the infectivity of dengue virus immune complexes. FcγRIIA is better equipped than FcγRIA to utilize alternative signaling pathways and entry mechanisms made available by relocation to such sites where weakly bound immune complexes might be more easily transferred to favorable entry pathways. After the immune complex is connected to FcyRIIa, the internalized FcyRIIa-immune complex enters the endosomal compartment, where the low affinity of FcyRIIa promotes the release of the immune complex. The attachment or dissociation of the FcγRIIa-immune complex enables innate immunity from antiviral to immunosuppression, induces the expression of IL-6, TNF and IL-10, and suppresses the expression of type I IFN (Rodrigo et al., 2006).
In vitro studies conducted in the monocyte cell lines U937 and K562 showed that FcγRII-mediated entry occurred through clathrin-coated vesicles, while FcγRI-mediated entry was not related to clathrin. In addition, since FcγRII translocates into lipid rafts after the immune complex is bound, entry through FcγRII is affected by membrane cholesterol levels (Carro, Piccini, & Damonte, 2018).
The activation of FcγR signaling can be blocked by the negative signaling of the inhibitory receptor FcγRIIb on the same cell surface. The destruction of tyrosine residues in ITIM or the removal of the poFcγRIIb cytoplasmic domain eliminates the ability of poFcγRII to mediate PRRSV-infected ADE (Wan, Chen, Li, Pang, & Bao, 2019). FcγRIIa promotes DENV-infected ADE, while FcγRIIb restricts it. By switching between these two isoforms containing the motifs of ITAM and ITIM, it was found that the intracellular part of FcγRII is the main determinant of ADE infection.
FcγRIII can not only significantly inhibit the levels of IFN-α and TNF-α mRNA, but also significantly increase the levels of IL-10 and viral mRNA during PRRSV viral infection. These clarify a mechanism of PRRSV-ADE regulated by poFcγRIII, which may be to increase virus internalization in its host cells and reduce antiviral cytokine levels to promote virus entry and replication (Gu et al., 2015; Zhang et al., 2016). FcγRIIIA promotes aggravation of dengue fever. The IgG1 subclass antibodies produced by DHF/DSS patients have a high affinity for the non-fucosylated Fc receptor FcγRIIIA, and the combination of the two induces platelet reduction, which is an important risk factor for thrombocytopenia syndrome, leads to the ADE effect of the disease (Wang, Sewatanon, et al., 2017).
3.2. Complement-mediated ADE
Complement dependent ADE is quite different from FcγR-dependent ADE, Complement can synergize with FCγR or cause ADE alone. High levels of CR2 and CD4 are required to enhance HIV-1 infection in T lymphocyt-like cell lines in the presence of subneutralizing levels of HIV-specific antibodies (Robinson, Montefiori, & Mitchell, 1990). In the absence of antibodies, complement CR3 and CR1 can promote HIV infection. Using antibodies to block CR1 or CR3 functions can completely inhibit complement-mediated enhancement (Thieblemont, 2010). Another independent pathway of complement mediated ADE in HIV-1 infection has been shown to require the complement component C1q. The IgG antibody binds closely to the viral epitope, so that the C1q binds to the Fc part of the antibody, and the immune complex binds to the C1q receptor on the cell surface (Prohászka et al., 1997). Similarly, C1q mediated ADE can enhance the infection of Ebola virus in non-monocytes by promoting the binding or endocytosis of the virus to the target cells (Takada, Feldmann, Ksiazek, & Kawaoka, 2003). Unlike FcR mediated intracellular signaling to promote ADE, C1q mediated ADE is only caused by the increase of virus particles attached to the cell surface (Byrne & Talarico, 2021; Furuyama, Nanbo, Maruyama, Marzi, & Takada, 2020). Acute and chronic inflammatory cardiomyopathies have been associated with a high prevalence of B19V DNA in endothelial cells of the myocardium. This may be related to the increased uptake of human parvovirus B19 on myocardial endothelial cells by complement factor C1q and its receptor CD93 mediated ADE. Despite its strong host tropism for erythroid progenitor cells, in the presence of specific antibodies to human parvovirus B19, complement dependent ADE increased the uptake of viral endothelial cells by 4000 times. ADE results in the accumulation of B19V DNA in the nucleus of infected cells, but it has no significant effect on gene expression and genome replication. The enhancement did not depend on the interaction between the virus antibody complex and FcR (von Kietzell et al., 2014).
3.3. Molecular signaling events in ADE
In addition to promoting the entry of viruses, ADE also affects the immune response of cells, inhibiting the antiviral activity of cells and thus promoting infection. The connection or dissociation of FcγR-immune complexes changed the innate response of antiviral and immunosuppression through unknown pathways, induced IL-6, TNF and IL-10, and inhibited the expression of type I IFN (Izmirly et al., 2020; Patro et al., 2019).
When immune complex entering the target cell can activates the negative regulator, dihydroxyacetone kinase (DAK) and autophagy-related 5 autophagy-related 12 (Atg5-Atg12), and then disrupts the RIG-I/MDA-5 signal cascade, down-regulating the downstream molecular signal (Ubol & Halstead, 2010; Ubol et al., 2010). Meanwhile, the connection between antibody complex and FcR downregulated TLRs gene expression, strongly stimulated the negative regulators of TIR-domain-containing adapter-inducing interferon-β (TRIF) and TNF receptor-associated factor 6 (TRAF-6), Sterile-alpha and Armadillo motif-containing protein (SARM), TRAF family member-associated NF-κB activator (TANK), negative regulators of the NF-κB pathway. Up-regulation of SARM and TANK results in down-regulation of TLR signaling molecules (Modhiran, Kalayanarooj, & Ubol, 2010; Taylor et al., 2015). But TLRs express upstream events and how SARM and TANK are activated is not entirely clear. These events lead to inhibition of innate responses mediated through the TLR signaling pathway and increased viral production. Both MyD88-dependent and non-dependent signaling molecules are downregulated during DENV-ADE infection (Kulkarni, 2020; Modhiran et al., 2010).
The connection between FcγRIIa and immune complex induced the activation of PI3K/PKB mediated by spleen tyrosine kinase (Syk). Activation of PI3K/PKB phosphorylates and inactivates glycogen synthase kinase (GSK)-3b, resulting in the observed high levels of IL-10. IL-10 can induce the cytokine response of Th2 and inhibit the production of proinflammatory cytokines, such as IFN-γ, IL-12, TNF-α and nitric oxide free radicals (Kuczera, Assolini, Tomiotto-Pellissier, Pavanelli, & Silveira, 2018). PRRSV ADE can up-regulate the production of IL10 through FcγRI and FcγRIII to promote the replication of PRRSV (Zhang et al., 2020).
IL-10 can effectively activate the SOCS system and inhibit Janus kinase/signal transduction and transcriptional activator (JAK/STAT) signal transduction pathways (Sukathida Ubol et al., 2010). The secreted IL10 cytokine binds to the IL10 receptor 1(IL10R1) on the membrane surface, and IL10R1 dimerizes with IL10R2 to play its downstream role. IL10R2 recruited the cytoplasmic protein Jak1, Phosphorylated STAT3 forms homodimers that are then transferred to the nucleus to promote transcriptional regulation of the target genes, thereby inhibiting iNOS gene expression and the production of nitric oxide free radicals (Kulkarni, 2020; Taylor et al., 2015). (As shown in Fig. 1 )

Host innate immune responses during ADE. (i) Dengue fever immune complex can enter target cells through FcR to activate negative regulators, dihydroxyacetone kinase (DAK) and autophagy-related 5 autophagy-related 12(Atg5-Atg12), thereby destroying the RIG-I / MDA-5 signaling pathway. Including beta interferon promoter stimulator 1 (IPS-1), inducible I-kappa B predominate kinase (IKKi), tumor necrosis factor receptor-associated factor 3(TRAF-3), and TANK-binding kinase 1 (TBK-I), etc Eventually inhibit type I IFN production. (ii) The expression of TLR-3, -4, -7 and TLR signal molecules was down regulated when the immune complex entered the target cells, resulting in inhibition of NF-κB activity and negative regulation of TLR signal transduction activity. (iii) The binding of leukocyte immunoglobulin like receptor B1 (LILRB1) with DENV virus inhibited the activation of Syk and eliminated the expression of ISG. Activated Syk upregulates IL10 via PI 3K/PKB, CREB signaling pathwayIL-10 stimulates the expression of SOCS3 and inactivates Janus kinase JAK-STAT signal, thus inhibiting the production of proinflammatory cytokines.IL-10 also inhibits the expression of nitric oxide synthase (iNOS) by inhibiting the activities of STAT-1 and IRF-1, thus inhibiting the production of nitric oxide (NO).
NO inhibits late viral protein synthesis and DNA synthesis, for example viral cysteine proteases are inactivated by NO-dependent s-nitrosoylation, NO inhibits PRRSV infection by cGMP-PKG dependent signaling (Saura et al., 1999; Zhang, Duan, Li, Zhao, & Xiao, 2016). The PBMC of DHF patients showed higher NS1 and lower NO serum level in acute fever period (Carvalho et al., 2014). The effect of NO is contradictory. NO is related to the severity of viral haemorrhagic fever. The increase of NO level in blood vessels will lead to the increase of vascular permeability and the damage of homeostasis in vivo. The higher concentration will affect the vascular tension and lead to the shock caused by virus (Thein et al., 2015). NO may play both protective and pathological roles, which depend on the concentration of NO.
The attachment of FcγRIIa to the DENV immune complex and the binding of LILRB1 to the DENV virions can synergistically induce ADE in THP-1 cells. (Cosman et al., 1997; Kulkarni, 2020). After Binding to LILRB1 the immunoreceptor tyrosine-based inhibition motif-bearing receptor recruits Src homology phosphatase-1(SHP-1) to dephosphorylate Syk, which leads to the decrease of ISG expression and the enhancement of DENV replication (Chan et al., 2014; Kulkarni, 2020).
In theory, DENV immune complex can use the inhibitory ITIM signal of FcγRIIb to destroy the expression of ISG. PoFcγRIIb mediated ADE pathway of PRRSV infection through recruitment of ship-1. The TBK-1-IRF-3-IFN-β signal transduction pathway was further inhibited to enhance PRRSV infection (Wan et al., 2019). In addition, the ability of poFcγRII to mediate PRRSV ADE can be eliminated by interrupting the tyrosine residues in ITIM or removing the cytoplasmic domain of Fc. The connection between immune complex and FcγRIIb can also weaken the activation of NF-κB triggered by TLR4. But ligation of FcγRIIb inhibits antibody-dependent enhancement of dengue virus infection. Antibodies help to form large viral aggregates, which in turn bind to FcγRIIb to inhibit the phagocytosis of monocytes (Chan et al., 2011). Whether there is ADE phenomenon may be related to the cell type and the concentration of antibodies. In cells with FcR, the production of cytokines mediated by FcγR depends on the proportion of activated and inhibited FcγR. Therefore, the simultaneous stimulation of activated and inhibited FcγR may not induce significant cytokine production.
Antibody-dependent DENV entry upregulates a host of host-dependent genes that support DENV infection. Chan found that some host-dependent factors were induced in primary monocytes under ADE conditions. ADE increases the spliceosome gene, so ADE may modify the splicing map of infected cells to produce an intracellular environment that is more conducive to virus replication and packaging. The higher expression of ribosome genes in ADE may explain the increased virus translation compared with DENV infection alone (Chan et al., 2019). In addition, it also includes DENV RNA binding protein, which requires for effective DENV amplification (Chan et al., 2019; Viktorovskaya, Greco, Cristea, & Thompson, 2016); ER-Golgi transport protein, which is necessary for viral transport; mitochondrial complex, which is required for ATP synthesis (Chan et al., 2019; Savidis et al., 2016).
3.4. Cellular compartmentalization
Cellular compartmentalization is used as a defense against human pathogens in both specialized and nonspecialized phagocytes. The septal boundary limits the entry of pathogens into the cytoplasm, and the expression of pattern recognition receptors is helpful for the detection and elimination of pathogens (Randow, Macmicking, & James, 2013).
In order to cope with the virus-resistant response triggered by the early activation of Fc, during ADE, the antibody-opsonized DENV was co-connected with the LILRB1 with ITIM to change the cellular compartment. Reduced Syk signal transduction not only leads to ISG-induced inhibition, but also downregulates the acidification of phagocytosis, enabling DENV to escape lysosomal degradation. SHP-1 is thought to inhibit phagocytic acidification by acting on membrane fusion during phagocytic transport. LILRB1 signal directly introduced the phagosome containing DENV into the area with lower acidification degree, thus preventing the rapid lysosomal degradation of DENV.
Similarly, inhibition of phagocytic acidification by lysosomal drugs has led to an increase in antibody-dependent infections, suggesting caution in the use of such drugs in the treatment of dengue (Jaume et al., 2011). Although the decrease or slowing down of phagocytic acidification during ADE may delay virus fusion and membrane removal, this may be a necessary trade-off, ultimately allowing DENV to escape lysosomal degradation, which is conducive to intracellular survival. During the ADE process of ACE2 mediated SARS-CoV, most of the virus particles that enter the cells through the ADE pathway are still trapped in the acid compartment and finally degraded. The inhibition of internal acidification can prevent them from degradation (Jaume et al., 2011; Ong et al., 2017).
The differential intracellular rate of fusion or compartmentalization could lead to exposure of the virus to a different repertoire of vesicular receptors, including pathogen recognition receptors, resulting in differential cellular responses (Chan et al., 2019). The differentiation compartmentalization of DENV may affect receptor interaction, viral replication and host response, which bring new research direction for the pathogenesis of virus.
4. Development of vaccine to eliminate ADE
Vaccine induced ADE has been found for a long time. Antibodies with poor affinity induced by formalin inactivated virus vaccines (e.g., RSV and measles) with Al adjuvant induced ADE during the initial infection of infants (Anderson et al., 2013). The development of vaccine to eliminate ADE mainly from two aspects: one is to mask or remove the antigen part that produces ADE, so that the antibody produced does not produce ADE effect; the other is to block the combination of antigen antibody complex and receptor, so as to inhibit Fc receptor or other receptor-mediated ADE.
4.1. Mask or remove the antigen part that produces ADE
Screaton developed a stable ZIKV E protein dimer vaccine that has no precursor membrane proteins and does not expose the immunodominant fusion loop epitope. Immunization of mice with ZIKV E dimers induces dimer-specific antibodies, which protect against ZIKV challenge during pregnancy. Importantly, the ZIKV E-dimer-induced response does not cross-react with DENV or induce ADE of DENV infection (Slon-Campos et al., 2019).
The cross reactive antibody of DENV and Zika virus is mainly induced by the DI/ DII region of E protein, especially the highly conserved fusionloop(fl)of DII region, which promotes DENV or ZIKV to enhance infection (Dejnirattisai et al., 2016; Priyamvada et al., 2016; Screaton, Mongkolsapaya, Yacoub, & Roberts, 2015). Lin showed that the enhanced infection of ij loop mutant of ZIKV E antigen in all four serotypes of DENV was greatly reduced, which indicated that ij loop mutant was a good target of glycan screening strategy, which could shield cross reactive antibody to induce ADE effect against DENV, and also retained the virus neutralization ability. Using ij cyclo-glycan to mask adenovirus vector, and then using DIII of E gene and FLIC of bacteria to express flic-diii fusion protein to enhance immunity, the results showed that the enhanced immunity by tetravalent DENV FLIC-DIII could improve the titer of neutralizing antibody and reduce ADE (Lin et al., 2019).
ZIKV-80E(N-terminal 80% of ZIKV E protein) expressed in yeast can self-assemble into nanoparticles. Nanoparticles without prM retain the antigenic integrity of neutralizing epitopes on E domain III (EDIII). Immunization of BALB/c mice can produce The ZIKV neutralizing antibody of this domain. The most exciting thing is that the antibodies induced by ZIKV-80E NPs did not induce the ADE potential of DENV and ZIKV in vivo (Shukla et al., 2020).
The use of reverse genetics to construct chimeric JEVpr/DENV2 with the Japanese encephalitis virus pr instead of the pr gene showed reduced virulence and good immunogenicity. In addition, anti-JEVpr/DENV2 sera showed extensive cross-reactivity and effective neutralizing activity against all four DENV serotypes and immature DENV2 (ImDENV2), and the ADEV activity of DENV decreased (Wang et al., 2015). Wang replaced DENV pr with JEV pr to construct a novel chimeric protein of JEV pr and DENV2 M peptide (JEVpr/DENV-M). Rabbit anti JEVpr/DENV-m had weak neutralization to both serotype DENV and immature DENV (ImDENV2), which did not change the neutralization ability of anti prM antibody, and did not cause the enhancement of infection of K562 cells with Fc receptor (Wang, Si, et al., 2017).
4.2. Block or interfere with the binding of the antigen-antibody complex to the receptor
Because of its own advantages that monoclonal antibody has become an important direction in the development of new vaccines. In particular, nano antibody does not contain Fc segment of common antibody, thus avoiding complement reaction caused by Fc domains and reducing ADE production. Palivizumab, an antiviral monoclonal antibody against respiratory syncytial virus (RSV) F protein, has significantly reduced RSV hospitalizations in premature infants and infants with chronic lung disease or congenital heart disease. Palizumab is clinically safe that fully demonstrates the development prospect of monoclonal antibody vaccine.
Wan analyzed the difference of ADE of coronavirus in different antibody doses, MERS MAb1 can inhibit virus entry at low concentration (by blocking DPP4 dependent entry pathway), promote virus entry at medium concentration (by enhancing CD32a dependent entry pathway), and inhibit virus entry at high concentration (by blocking DPP4 and CD32a dependent entry pathway), which can also explain why virus ADE only exists at some concentrations, and neither too high nor too low can induce virus entry ADE (Wan et al., 2020).
Widjaja identified eight monoclonal antibodies that bind to non-overlapping epitopes on MERS-S with high affinity and interfere with the three known functions of viral proteins: sialic acid binding, receptor binding, and membrane fusion (Widjaja et al., 2019). At low doses, these antibodies protected mice from the deadly MERS-CoV infection. It is worth noting that there are two mAbs (G2 and G4) do not interfere with receptor binding, but abolish intercellular fusion, which means that they can interfere with spike mediated membrane fusion by blocking the conformational changes required for fusion. The high protective mAb showed only moderate neutralization activity in vitro, indicating that strong neutralization activity was not a prerequisite for protection. This provides us with a huge hint to develop a vaccine to eliminate ADE.
The development of new methods to deliver cross-reactive, neutralizing monoclonal antibodies to the circulatory system could provide rapid, comprehensive and inexpensive protection against related diseases. Flingai using monoclonal antibody DNA encoding antibody (DMAb) encodes for an Fc region-modified with abrogated FcγR binding by way of two leucine-to-alanine (LALA) mutations in the CH2 region and eliminate antibody-dependent enhancement of DENV(Beltramello et al., 2010; Flingai et al., 2015). By delivering multiple DENV DMAb plasmids, this increases human IgG levels and the number of targeted serotypes. Injecting the DNA that encodes the antibody produces biologically relevant levels of monoclonal antibodies. The protection provided by DMAb is significantly faster than vaccination protection that takes weeks or months to reach peak effect. Similarly, Elliott uses DMAb technology to encode two monoclonal antibodies with broad cross-protection against influenza A and B (Elliott et al., 2017). It can provide mice with extensive protection against influenza A and B infections.
Zhang made specific antigen peptides, linkers and Fc-III mimetic peptides into Dual-functional Conjugate of Antigenic peptide and Fc-III tag(DCAF) (Zhang et al., 2019). The Fc-III part inhibited the dengue fever ADE process to a certain extent and blocked the antibodies-Virus or antibody-Fc receptor interaction. At the cell level in vitro, DCAF successfully inhibited the ADE effect of dengue fever type 2, indicating that reducing the affinity of Fc region binding to Fc receptor can weaken ADE.
Wang produced anti-idiotypic antibodies against prM antibodies that can cause ADE, and found that they can prevent ADE not only in vitro but also in vivo (Wang et al., 2017). After mice immunized with prM mAb-specific anti-idiotypic antibodies (prM-AID) were infected with DENV-1, interleukin 10 (IL-10) and alanine aminotransferase (ALT) were as low as the negative control level, and the number of platelets increased significantly compared with the control group. It suggesting that anti-idiotypic antibodies may be a new option for treating ADE caused by DENV infection.
Influenza M2 protein extracellular functional area (M2e) has a high degree of conservation which can provide a wide range of protective effects. Antibodies to the influenza M2e protein are well protected by blocking viral ion channel function and mediating ADCC or ADPC action to clear infected cells. Vlieger prepared VHH antibodies against M2e and mouse FcRIV (De Vlieger et al., 2019). Specific antibodies that target only FcRIV may be an advantage because they avoid inhibiting FcRIIb receptor activation in this way. Intranasally administered bi-specific VHH fusion antibodies selectively bind to M2e on infected target cells and activate FcRIV to resist influenza A virus attack. Since antibodies against M2e do not work by neutralizing the virus, this greatly reduces the possibility of ADE. However, the antibody effect of M2e immunization alone is limited, and the development may need to cooperate with neutralization effect for HA to provide a higher protective effect (Wei et al., 2020).
FcγR is highly sensitive to N-linked glycosylation mode of Fc region. Therefore, unique plant N-glycans may affect the characteristics of mAbs produced, including ADE activity (Sun, Chen, & Lai, 2017). E60 (mE60) produced by mammalian cells showed ADE in vivo and in vitro when DENV was infected (Balsitis et al., 2010). E60 produced in plants showed a single predominant expected N-glycoform type with high homogeneity. These E60 glycovariants maintain specific binding to EDII antigen with a kinetics similar to mE60, and showing neutralization activity against a variety of DENV serotypes. Most importantly, E60 produced in plants forwent ADE activity in K562 cells expressing FcγR (Matthew et al., 2016).
5. Effect of adjuvant and inactivation selection on ADE
The choice of adjuvant will also affect the immune effect. The carbonyl group on the vaccine antigen treated with formaldehyde can enhance the T-helper 2 (Th 2) reaction and enhance the respiratory syncytial virus (RSV) disease in mice, which can be partially reversed by the chemical reduction of the carbonyl group (Delgado et al., 2009; Moghaddam et al., 2006; Ubol & Halstead, 2010).
However, inactivation of RSV by methods other than formalin also makes experimental animals more sensitive to disease. RSV-immunized mice treated with UV-irradiated inactivated purified fusion protein or vaccinia virus RSV replicas developed enhanced diseases after being challenged by wild-type viruses (Delgado et al., 2009; Olszewska, Suezer, Sutter, & Openshaw, 2004). Inactivated vaccines have insufficient TLR activation, and adding TLR agonists can prevent ERD. Speculate that this may be related to adjuvant.
Boelen found that unimmunized mice infected with RSV showed a Th1 cell response with mild symptoms; but FI-RSV (formalin inactivated antigen with AL adjuvant added) immunization resulted in aggravated lung histopathology and showed obvious Th2 cellular immune response, although the vaccine is still partially protective, can induce low titer neutralizing antibody response, and virus replication is reduced; Fi-mock (Formalin Inactivated Cell Antigen Negative Control) immunized mice showed increased RSV replication, also manifested by a significant Th2 cell immune response (Boelen et al., 2000). This shows that the Th2 cell immune response induced by the formalin-inactivated vaccine with Al adjuvant seems to be conducive to RSV replication. The combination of Montanide ISA-51 and CpG ODN (oligodeoxynucleotide) has been used in the development of coronavirus vaccine, which can synergistically enhance Th1 immune response (Gupta & Gupta, 2020). Immunization of mice and cynomolgus monkeys with Montanide ISA-51 and CpG emulsified SARS-CoV recombinant N protein can cause a strong Th1 immune response (Liu et al., 2006).
6. Conclusions and future prospects
ADE has been proven in vitro and in animal models for a variety of viruses including DENV, HIV, RSV, SARS-CoV and WNV (Table 1 ).
Table 1
Key characteristics of antibody-dependent enhancement (ADE) infected by different viruses.
| Virus | Main Host | Types of ADE | Clinical manifestations of ADE | Enhancing epitopes location | Impact of vaccine application |
|---|---|---|---|---|---|
| Dengue virus | Humans,Primate,Aedes | 1.FcR mediated virus-antibody immune complexes infect monocytes, macrophages, and dendritic cells 2.Through FcR, LILR-B1 regulates the host’s antiviral response, inhibits the innate response mediated by the TLR signaling pathway, disrupts the RIG-I/MDA-5 signal cascade, and induces IL-10 production | 1. Increased susceptibility to other serotypes 2. Increased viral infection and association with severe dengue fever (DHF/DSS) 3. Infants born to dengue fever-immunized mothers, serious diseases that may be infected when maternal antibodies are reduced | prM protein, E protein DII-FL region | Vaccine raises the risk of ADE for DENV infection.Sero-negative dengue vaccinators are at increased risk of severe dengue, and WHO recommends vaccination only for sero-positive dengue |
| SRAS-CoV | Rhinolophus sinicus,Paguma larvatas,Humans | FcR-ADE(mainly mediated by FcγRII) | May be related to severe lymphopenia | Spike protein | Infection after immunization may cause severe acute lung injury (ALI) |
| Influenza virus | Humans,Pigs,Birds, Ferrets | FcR-ADE | Increased risk of a (H1N1) pdm09 disease | HA,NA | 1.Trivalent inactivated influenza vaccine (TIV) in 2008-09 increased risk of a (H1N1) pdm09 disease 2. The vaccine may be associated with vaccine associated enhanced respiratory disease (VAERD) |
| Porcine Reproductive and Respiratory Syndrome | Pigs | FcR-ADE (Including FcγRI,FcγRII,FcγRIII, FCɛRI) | Promote virus infection and enhance clinical symptoms, Increase the level and duration of viremia | GP5,N Protein | Infected with prrsv after immunization with inactivated vaccine, clinical symptoms increased |
| Human immunodeficiency virus | Humans | 1.FcR-ADE (Including FcγRI,FcγRII,FcγRIII,FCαR) , FcR promotes virus entry by enhancing adhesion to CD4 receptor 2.CR3, C1q complement mediated ADE | 1.ADE and plasma viral load is positive correlation. ADE accelerates immunosuppression and disease progression 2.Enhancing antibodies is beneficial to the emergence of ADE susceptible mutants | N-terminal immune dominant domain of gp41,gp120 | One of the factors affecting vaccine development,higher rates of infection/risk among vaccinees were observed in RV144 clinical trials, but have not been confirmed to be directly associated with ADE |
| West nile virus | Horses,Humans,Birds | 1.Fcγ receptor dependent ADE 2.CR3 dependent ADE | Increased viral infectivity | Domain I and domain II of the E protein | No vaccine has been marketed. Plasma samples of human WNV infection during rehabilitation can enhance ZIKV infection in vitro and in vivo |
| Respiratory syncytial virus | Humans | FcR mediate the uptake of viruses into monocytes, macrophages, and dendritic cells, leading to enhanced infection | ADE infection leads to activation of Th2 response and increased expression of TNF-α and IL-6, resulting in aggravated disease ADE infection of lung dendritic cells (DCs) can negatively regulate the function of DC cells, resulting in impaired T cell activation | Glycoproteins G and F | Formalin inactivated RSV vaccine recipients have increased disease and even led to death |
The essence of ADE effect is a part of immune regulation. In the next step, we need to clarify the mechanism of ADE and clarify the conditions of ADE occurrence, find out factors that regulate Syk activation and inhibition, the balance between antiviral and inflammatory responses, and upregulation of immunosuppressive IL-10, so as to better block the occurrence of ADE from the perspective of iADE. This will provide ideas and directions for the development of new drugs for infectious diseases such as HIV, DENV, influenza, and even SARS CoV2.
Influenza, SARS-CoV, and PRRSV vaccines all have the potential to cause ADE, or to cause vaccine-related enhanced respiratory disease (VAERD). Although some cannot be directly attributed to ADE, it cannot be denied that ADE is very likely to be one of the potential factors. From the experience of SARS-CoV vaccine development, the development of COIVD-19 vaccines should pay attention to ADE. In the future, it is necessary to identify the antigen epitopes of ADE induced by viruses, effectively avoid them in vaccine design, and further discover effective virus neutralizing epitopes and other non-neutralizing but protective epitopes, which will guide the future design of protective vaccines, therapeutic monoclonal antibodies and nano antibodies.
Activation of the immune system increases the number of activated CD4 (+) T cells, which are more susceptible to infection than inactive T cells (Bukh et al., 2014). Human CMV infection may lead to increased susceptibility to HIV-1 infection. HCMV can preventing antiviral IFN-stimulated gene production by degradation of JAK1, interfering with STAT signaling (Johnson et al., 2018; Miller & Boss, 1998; Paulus, Krauss, & Nevels, 2006). This is similar to the partially suppressed signal produced by ADE. For the outbreak of COVID-19 worldwide, it is speculated that those individuals with severe illness may have been exposed to one or more previous coronaviruses and are experiencing antibody-dependent enhancement (Tetro, 2020). iADE suppresses the function of the immune system and promotes viral infection or disease. Future research should pay attention to this aspect, that is, whether it will cause the increase of heterologous disease infection when in iADE.
In the experience of RSV vaccine design, especially in the selection of adjuvants in recombinant subunit vaccines, Th1 biased adjuvants should be selected as much as possible, such as poly(I:C) (TLR3),AS04 (TLR4), imiquimod (TLR7/8 ), CpG motifs (TLR9), monophosphoryl lipid A (MPL), and avoid the selection of Th2 biased adjuvants, such as Al adjuvant (Apostolico Jde, Lunardelli, Coirada, Boscardin, & Rosa, 2016; Gupta & Gupta, 2020; Lindblad, 2004). In the future, the preparation of vaccines also needs to develop new safe and efficient adjuvants to avoid the generation of ADE. Kabir proves that ADE can greatly increase the incidence of new diseases. For multiple serotype infectious diseases such as dengue fever, joint vaccine is more effective. The primary vaccine is a better control tool than the secondary vaccine. In order to suppress secondary infections, secondary vaccinations require high efficiency (Kabir & Tanimoto, 2020). This may be because secondary infections are located further downstream in the dynamic process of infection and require a greater effect to suppress the spread of secondary infections. More rigorous, reasonable and safe immune procedures are also important issues to be solved in future vaccine development.
Acknowledgments
Support for this work was received from the Science Fundation for Distinguished Young Scholars of Shaanxi Province (2021JC-18), the Open Project of the State Key Laboratory of Veterinary Etiological Biology (SKLVEB2020KFKT017), the Youth Innovation Team of Shaanxi Universities, the Science and Technology Extension Project in Northwest A&F University (TGZX2020-24) the Fundamental Research Funds for the Central Universities (2452021154).Go to:
References
- Anderson L.J., Dormitzer P.R., Nokes D.J., Rappuoli R., Roca A., Graham B.S. Strategic priorities for respiratory syncytial virus (RSV) vaccine development. Vaccine. 2013;31(Suppl 2):B209–B215. doi: 10.1016/j.vaccine.2012.11.106. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Apostolico Jde S., Lunardelli V.A., Coirada F.C., Boscardin S.B., Rosa D.S. Adjuvants: Classification, modus operandi, and licensing. Journal of Immunology Research. 2016;2016:1459394. doi: 10.1155/2016/1459394. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Arvin A.M., Fink K., Schmid M.A., Cathcart A., Spreafico R., Havenar-Daughton C. A perspective on potential antibody-dependent enhancement of SARS-CoV-2. Nature. 2020;584(7821):353–363. doi: 10.1038/s41586-020-2538-8. [PubMed] [CrossRef] [Google Scholar]
- Arvin A.M., Fink K., Schmid M.A., Cathcart A., Virgin H.W. A perspective on potential antibody-dependent enhancement of SARS-CoV-2. Nature. 2020:1–14. [PubMed] [Google Scholar]
- Balsitis S.J., Williams K.L., Lachica R., Flores D., Kyle J.L., Mehlhop E. Lethal antibody enhancement of dengue disease in mice is prevented by Fc modification. PLoS Pathogens. 2010;6(2) doi: 10.1371/journal.ppat.1000790. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Beltramello M., Williams K.L., Simmons C.P., Macagno A., Simonelli L., Quyen N.T. The human immune response to Dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activity. Cell Host & Microbe. 2010;8(3):271–283. doi: 10.1016/j.chom.2010.08.007. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Boelen A., Andeweg A., Kwakkel J., Lokhorst W., Bestebroer T., Dormans J. Both immunisation with a formalin-inactivated respiratory syncytial virus (RSV) vaccine and a mock antigen vaccine induce severe lung pathology and a Th2 cytokine profile in RSV-challenged mice. Vaccine. 2000;19(7):982–991. [PubMed] [Google Scholar]
- Bukh I., Calcedo R., Roy S., Carnathan D.G., Grant R., Qin Q. Increased mucosal CD4 + T cell activation in rhesus macaques following vaccination with an adenoviral vector. Journal of Virology. 2014;88(15):8468–8478. doi: 10.1128/jvi.03850-13. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Byrne A.B., Talarico L.B. Role of the complement system in antibody-dependent enhancement of flavivirus infections. International Journal of Infectious Diseases. 2021;103:404–411. [PubMed] [Google Scholar]
- Cancel-Tirado S.M., Evans R.B., Yoon K.J. Monoclonal antibody analysis of porcine reproductive and respiratory syndrome virus epitopes associated with antibody-dependent enhancement and neutralization of virus infection. Veterinary Immunology and Immunopathology. 2004;102(3):249–262. doi: 10.1016/j.vetimm.2004.09.017. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Carro A.C., Piccini L.E., Damonte E.B. Blockade of dengue virus entry into myeloid cells by endocytic inhibitors in the presence or absence of antibodies. PLoS Neglected Tropical Diseases. 2018;12(8) doi: 10.1371/journal.pntd.0006685. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Carvalho D.M., Garcia F.G., Terra A.P., Lopes Tosta A.C., Silva Lde A., Castellano L.R. Elevated dengue virus nonstructural protein 1 serum levels and altered toll-like receptor 4 expression, nitric oxide, and tumor necrosis factor alpha production in dengue hemorrhagic Fever patients. Journal of Tropical Medicine. 2014;2014:901276. doi: 10.1155/2014/901276. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Chan C.Y.Y., Low J.Z.H., Gan E.S., Ong E.Z., Zhang S.L., Tan H.C. Antibody-dependent dengue virus entry modulates cell intrinsic responses for enhanced infection. mSphere. 2019;4(5) doi: 10.1128/mSphere.00528-19. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Chan K.R., Ong E.Z., Tan H.C., Zhang S.L., Zhang Q., Tang K.F. Leukocyte immunoglobulin-like receptor B1 is critical for antibody-dependent dengue. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(7):2722–2727. doi: 10.1073/pnas.1317454111. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Chan K.R., Zhang S.L.-X., Tan H.C., Chan Y.K., Chow A., Lim A.P.C. Ligation of Fc gamma receptor IIB inhibits antibody-dependent enhancement of dengue virus infection. Proceedings of the National Academy of Sciences. 2011 [PMC free article] [PubMed] [Google Scholar]
- Chareonsirisuthigul T., Kalayanarooj S., Ubol S. Dengue virus (DENV) antibody-dependent enhancement of infection upregulates the production of anti-inflammatory cytokines, but suppresses anti-DENV free radical and pro-inflammatory cytokine production, in THP-1 cells. The Journal of General Virology. 2007;88(Pt 2):365–375. doi: 10.1099/vir.0.82537-0. [PubMed] [CrossRef] [Google Scholar]
- Chau T.N., Hieu N.T., Anders K.L., Wolbers M., Lien le B., Hieu L.T. Dengue virus infections and maternal antibody decay in a prospective birth cohort study of Vietnamese infants. The Journal of Infectious Diseases. 2009;200(12):1893–1900. doi: 10.1086/648407. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Chau T.N., Quyen N.T., Thuy T.T., Tuan N.M., Hoang D.M., Dung N.T. Dengue in Vietnamese infants—results of infection-enhancement assays correlate with age-related disease epidemiology, and cellular immune responses correlate with disease severity. The Journal of Infectious Diseases. 2008;198(4):516–524. doi: 10.1086/590117. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Chen Z., Zhang L., Qin C., Ba L., Yi C.E., Zhang F. Recombinant modified vaccinia virus Ankara expressing the spike glycoprotein of severe acute respiratory syndrome coronavirus induces protective neutralizing antibodies primarily targeting the receptor binding region. Journal of Virology. 2005;79(5):2678–2688. doi: 10.1128/JVI.79.5.2678-2688.2005. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Cheung C.Y., Poon L.L., Ng I.H., Luk W., Sia S.F., Wu M.H. Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: Possible relevance to pathogenesis. Journal of Virology. 2005;79(12):7819–7826. doi: 10.1128/JVI.79.12.7819-7826.2005. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Coish J.M., MacNeil A.J. Out of the frying pan and into the fire? Due diligence warranted for ADE in COVID-19. Microbes and Infection. 2020 doi: 10.1016/j.micinf.2020.06.006. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Cosman D., Fanger N., Borges L., Kubin M., Chin W., Peterson L. A novel immunoglobulin superfamily receptor for cellular and viral MHC class I molecules. Immunity. 1997;7(2):273. [PubMed] [Google Scholar]
- Cui G., Si L., Wang Y., Zhou J., Yan H., Jiang L. Antibody-dependent enhancement (ADE) of dengue virus: Identification of the key amino acid that is vital in DENV vaccine research. The Journal of Gene Medicine. 2021;23(2) [PMC free article] [PubMed] [Google Scholar]
- de Taeye S.W., Rispens T., Vidarsson G. The ligands for human IgG and their effector functions. Antibodies (Basel) 2019;8(2) doi: 10.3390/antib8020030. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- De Vlieger D., Hoffmann K., Van Molle I., Nerinckx W., Van Hoecke L., Ballegeer M. Selective engagement of FcgammaRIV by a M2e-specific single domain antibody construct protects against influenza A virus infection. Frontiers in Immunology. 2019;10:2920. doi: 10.3389/fimmu.2019.02920. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Dejnirattisai W., Supasa P., Wongwiwat W., Rouvinski A., Barba-Spaeth G., Duangchinda T. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nature Immunology. 2016;17(9):1102–1108. doi: 10.1038/ni.3515. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Delgado M.F., Coviello S., Monsalvo A.C., Melendi G.A., Hernandez J.Z., Batalle J.P. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nature Medicine. 2009;15(1):34–41. doi: 10.1038/nm.1894. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Elliott S.T.C., Kallewaard N.L., Benjamin E., Wachter-Rosati L., McAuliffe J.M., Patel A. DMAb inoculation of synthetic cross reactive antibodies protects against lethal influenza A and B infections. NPJ Vaccines. 2017;2:18. doi: 10.1038/s41541-017-0020-x. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Flingai S., Plummer E.M., Patel A., Shresta S., Mendoza J.M., Broderick K.E. Protection against dengue disease by synthetic nucleic acid antibody prophylaxis/immunotherapy. Scientific Reports. 2015;5:12616. doi: 10.1038/srep12616. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Furuyama W., Marzi A., Carmody A.B., Maruyama J., Kuroda M., Miyamoto H. Fcγ-receptor IIa-mediated Src Signaling Pathway Is Essential for the Antibody-Dependent Enhancement of Ebola Virus Infection. PLoS Pathogens. 2016;12(12) doi: 10.1371/journal.ppat.1006139. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Furuyama W., Nanbo A., Maruyama J., Marzi A., Takada A. A complement component C1q-mediated mechanism of antibody-dependent enhancement of Ebola virus infection. PLoS Neglected Tropical Diseases. 2020;14(9) doi: 10.1371/journal.pntd.0008602. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Gao Q., Bao L., Mao H., Wang L., Xu K., Yang M. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369(6499):77–81. doi: 10.1126/science.abc1932. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Garg H., Yeh R., Watts D.M., Mehmetoglu-Gurbuz T., Resendes R., Parsons B. Enhancement of Zika virus infection by antibodies from West Nile virus seropositive individuals with no history of clinical infection. BMC Immunology. 2021;22(1):5. doi: 10.1186/s12865-020-00389-2. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Goncalvez A.P., Engle R.E., Claire M.S., Purcell R.H., Lai C.-J. Monoclonal antibody-mediated enhancement of dengue virus infection in vitro and in vivo and strategies for prevention. Proceedings. National Academy of Sciences. United States of America. 2007;104(22):9422–9427. [PMC free article] [PubMed] [Google Scholar]
- Gordon M.J.C.J.S.P.S. Complement receptor mediates enhanced flavivirus replication in macrophages. Journal of Experimental Medicine. 1983;158(1):258–263. [PMC free article] [PubMed] [Google Scholar]
- Gu W., Guo L., Yu H., Niu J., Huang M., Luo X. Involvement of CD16 in antibody-dependent enhancement of porcine reproductive and respiratory syndrome virus infection. The Journal of General Virology. 2015;96(Pt 7):1712–1722. doi: 10.1099/vir.0.000118. [PubMed] [CrossRef] [Google Scholar]
- Guilliams M., Bruhns P., Saeys Y., Hammad H., Lambrecht B.N. The function of Fcgamma receptors in dendritic cells and macrophages. Nature Reviews. Immunology. 2014;14(2):94–108. doi: 10.1038/nri3582. [PubMed] [CrossRef] [Google Scholar]
- Gupta T., Gupta S.K. Potential adjuvants for the development of a SARS-CoV-2 vaccine based on experimental results from similar coronaviruses. International Immunopharmacology. 2020;86:106717. doi: 10.1016/j.intimp.2020.106717. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Halstead S.B., O’Rourke E.J., Allison A.C. Dengue viruses and mononuclear phagocytes. II. Identity of blood and tissue leukocytes supporting in vitro infection. The Journal of Experimental Medicine. 1977;146:218–229. [PMC free article] [PubMed] [Google Scholar]
- Halstead S.B., Chow J.S., Marchette N.J. Immunological enhancement of dengue virus replication. Nature: New Biology. 1973;243(122):24–26. [PubMed] [Google Scholar]
- Halstead S.B., Mahalingam S., Marovich M.A., Ubol S., Mosser D.M. Intrinsic antibody-dependent enhancement of microbial infection in macrophages: Disease regulation by immune complexes. Lancet Infectious Diseases. 2010;10(10):712–722. [PMC free article] [PubMed] [Google Scholar]
- Hanson Q.M., Barb A.W. A perspective on the structure and receptor binding properties of immunoglobulin G Fc. Biochemistry. 2015;54(19):2931–2942. doi: 10.1021/acs.biochem.5b00299. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Heinz F.X., Stiasny K. Flaviviruses and their antigenic structure. Journal of Clinical Virology the Official Publication of the Pan American Society for Clinical Virology. 2012;55(4):289–295. [PubMed] [Google Scholar]
- Hermanns K., Gohner C., Kopp A., Schmidt A., Merz W.M., Markert U.R. Zika virus infection in human placental tissue explants is enhanced in the presence of dengue virus antibodies in-vitro. Emerging Microbes & Infections. 2018;7(1):198. doi: 10.1038/s41426-018-0199-6. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Ho M.-S., Chen W.-J., Chen H.-Y., Lin S.-F., Wang M.-C., Di J. Neutralizing antibody response and SARS severity. Emerging Infectious Diseases. 2005;11(11):1730–1737. [PMC free article] [PubMed] [Google Scholar]
- Izmirly A.M., Alturki S.O., Alturki S.O., Connors J., Haddad E.K. Challenges in dengue vaccines development: Pre-existing infections and cross-reactivity. Frontiers in Immunology. 2020;11 doi: 10.3389/fimmu.2020.01055. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Janoff E.N., Wahl S.M., Kelly T., Smith P.D. Modulation of human immunodeficiency virus type 1 infection of human monocytes by IgA. Journal of Infectious Diseases. 1995;3:3. [PubMed] [Google Scholar]
- Jaume M., Yip M.S., Cheung C.Y., Leung H.L., Li P.H., Kien F. Anti-severe acute respiratory syndrome coronavirus spike antibodies trigger infection of human immune cells via a pH- and cysteine protease-independent FcgammaR pathway. Journal of Virology. 2011;85(20):10582–10597. doi: 10.1128/JVI.00671-11. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Johnson L., Johnson E.L., Boggavarapu S., Johnson E.S., Lal A.A., Agrawal P. Human cytomegalovirus enhances placental susceptibility and replication of human immunodeficiency virus type 1 (HIV-1), which may facilitate in utero HIV-1 transmission. The Journal of Infectious Diseases. 2018 [PMC free article] [PubMed] [Google Scholar]
- Kabir K.M.A., Tanimoto J. Cost-efficiency analysis of voluntary vaccination against n-serovar diseases using antibody-dependent enhancement: A game approach. Journal of Theoretical Biology. 2020;503:110379. doi: 10.1016/j.jtbi.2020.110379. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Karl-Klaus C., Nico V.…and Molecular Characterization of Porcine Reproductive and Respiratory Syndrome Virus, a Member of the Arterivirus Group. Virology. 1993 [PMC free article] [PubMed] [Google Scholar]
- Kliks S.C., Nimmanitya S., Nisalak A., Burke D.S. Evidence that maternal antibodies are important in the development of dengue hemorrhagic fever in infants. The American Journal of Tropical Medicine and Hygiene. 1988;38(2):411–419. [PubMed] [Google Scholar]
- Kuczera D., Assolini J.P., Tomiotto-Pellissier F., Pavanelli W.R., Silveira G.F. Highlights for Dengue Immunopathogenesis: Antibody-Dependent Enhancement, Cytokine Storm, and Beyond. Journal of Interferon & Cytokine Research. 2018;38(2):69–80. [PubMed] [Google Scholar]
- Kulkarni R. Dynamics of Immune Activation in Viral Diseases. 2020. Antibody-Dependent Enhancement of Viral Infections; pp. 9–41. [Google Scholar]
- Lafferty R.A.H.A.K.J. The Enhancement of Virus Infectivity by Antibody. Virology. 1967;33:250–261. [PubMed] [Google Scholar]
- Langerak T., Mumtaz N., Tolk V.I., van Gorp E.C.M., Martina B.E., Rockx B. The possible role of cross-reactive dengue virus antibodies in Zika virus pathogenesis. PLoS Pathogens. 2019;15(4) doi: 10.1371/journal.ppat.1007640. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Li D., Edwards R.J., Manne K., Martinez D.R., Saunders K.O. In vitro and in vivo functions of SARS-CoV-2 infection-enhancing and neutralizing antibodies. Cell. 2021 [PMC free article] [PubMed] [Google Scholar]
- Li Y., Xu G., Du X., Xu L., Ma Z., Li Z. Genomic characteristics and pathogenicity of a new recombinant strain of porcine reproductive and respiratory syndrome virus. Archives of Virology. 2021;166(2):389–402. doi: 10.1007/s00705-020-04917-8. [PubMed] [CrossRef] [Google Scholar]
- Lin H.H., Yang S.P., Tsai M.J., Lin G.C., Wu H.C., Wu S.C. Dengue and Zika Virus Domain III-Flagellin Fusion and Glycan-Masking E Antigen for Prime-Boost Immunization. Theranostics. 2019;9(16):4811–4826. doi: 10.7150/thno.35919. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Lindblad E.B. Aluminium compounds for use in vaccines. Immunology and Cell Biology. 2004;82(5):497–505. doi: 10.1111/j.0818-9641.2004.01286.x. [PubMed] [CrossRef] [Google Scholar]
- Littaua R.A., Kurane I., Ennis F.A. Human IgG Fc receptor II mediates antibody dependent enhancement of dengue virus infection. Journal of Immunology. 1990;144(8):3183–3186. [PubMed] [Google Scholar]
- Liu S.J., Leng C.H., Lien S.P., Chi H.Y., Huang C.Y., Lin C.L. Immunological characterizations of the nucleocapsid protein based SARS vaccine candidates. Vaccine. 2006;24(16):3100–3108. doi: 10.1016/j.vaccine.2006.01.058. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Liu Y., Soh W.T., Kishikawa J.I., Hirose M., Nakayama E.E., Li S. An infectivity-enhancing site on the SARS-CoV-2 spike protein targeted by antibodies. Cell. 2021;184(13) doi: 10.1016/j.cell.2021.05.032. 3452–3466.e3418. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Liu L., Wei Q., Lin Q., Fang J., Wang H., Kwok H. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight. 2019;4(4) doi: 10.1172/jci.insight.123158. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Lok S.M. The Interplay of Dengue Virus Morphological Diversity and Human Antibodies. Trends in Microbiology. 2016;24(4):284–293. doi: 10.1016/j.tim.2015.12.004. [PubMed] [CrossRef] [Google Scholar]
- Lv H., Wu N.C., Tsang O.T., Yuan M., Perera R., Leung W.S. Cross-reactive antibody response between SARS-CoV-2 and SARS-CoV infections. bioRxiv. 2020 doi: 10.1101/2020.03.15.993097. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Matthew D., Jonathan H., Paul A.M., Sun H., Lai H., Ming Y. Plant-produced anti-dengue virus monoclonal antibodies exhibit reduced antibody-dependent enhancement of infection activity. Journal of General Virology. 2016;97(12):3280–3290. [PMC free article] [PubMed] [Google Scholar]
- Miller R., Boss Human cytomegalovirus inhibits major histocompatibility complex class II expression by disruption of the Jak/Stat pathway. Journal of Experimental Medicine. 1998 [PMC free article] [PubMed] [Google Scholar]
- Modhiran N., Kalayanarooj S., Ubol S. Subversion of innate defenses by the interplay between DENV and pre-existing enhancing antibodies: TLRs signaling collapse. PLoS Neglected Tropical Diseases. 2010;4(12) doi: 10.1371/journal.pntd.0000924. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Moghaddam A., Olszewska W., Wang B., Tregoning J.S., Helson R., Sattentau Q.J. A potential molecular mechanism for hypersensitivity caused by formalin-inactivated vaccines. Nature Medicine. 2006;12(8):905–907. doi: 10.1038/nm1456. [PubMed] [CrossRef] [Google Scholar]
- Morens D.M., Larsen L.K., Halstead S.B. Study of the distribution of antibody-dependent enhancement determinants on dengue 2 isolates using dengue 2-derived monoclonal antibodies. Journal of Medical Virology. 1987;22(2):163–167. [PubMed] [Google Scholar]
- Morrone S.R., Lok S.M. Structural perspectives of antibody-dependent enhancement of infection of dengue virus. Current Opinion in Virology. 2019;36:1–8. doi: 10.1016/j.coviro.2019.02.002. [PubMed] [CrossRef] [Google Scholar]
- Nimmerjahn F., Bruhns P., Horiuchi K., Ravetch J.V. FcγRIV: A Novel FcR with Distinct IgG Subclass Specificity. Immunity. 2005;23(1):41–51. doi: 10.1016/j.immuni.2005.05.010. [PubMed] [CrossRef] [Google Scholar]
- Oliveira R.A., de Oliveira-Filho E.F., Fernandes A.I., Brito C.A., Marques E.T., Tenorio M.C. Previous dengue or Zika virus exposure can drive to infection enhancement or neutralisation of other flaviviruses. Memórias do Instituto Oswaldo Cruz. 2019;114 doi: 10.1590/0074-02760190098. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Olszewska W., Suezer Y., Sutter G., Openshaw P.J. Protective and disease-enhancing immune responses induced by recombinant modified vaccinia Ankara (MVA) expressing respiratory syncytial virus proteins. Vaccine. 2004;23(2):215–221. doi: 10.1016/j.vaccine.2004.05.015. [PubMed] [CrossRef] [Google Scholar]
- Ong E.Z., Zhang S.L., Tan H.C., Gan E.S., Chan K.R., Ooi E.E. Dengue virus compartmentalization during antibody-enhanced infection. Scientific Reports. 2017;7:40923. doi: 10.1038/srep40923. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Patro A.R.K., Mohanty S., Prusty B.K., Singh D.K., Gaikwad S., Saswat T. Cytokine signature associated with disease severity in dengue. Viruses. 2019;11(1) doi: 10.3390/v11010034. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Paul L.M., Carlin E.R., Jenkins M.M., Tan A.L., Barcellona C.M., Nicholson C.O. Dengue virus antibodies enhance Zika virus infection. Clinical & Translational Immunology. 2016;5(12) doi: 10.1038/cti.2016.72. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Paulus C., Krauss S., Nevels M. A human cytomegalovirus antagonist of type I IFN-dependent signal transducer and activator of transcription signaling. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(10):3840–3845. doi: 10.1073/pnas.0600007103. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Priyamvada L., Quicke K.M., Hudson W.H., Onlamoon N., Sewatanon J., Edupuganti S. Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(28):7852–7857. doi: 10.1073/pnas.1607931113. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Prohászka Z., Nemes J., Hidvégi T., Tóth F.D., Kerekes K., Erdei A. Two parallel routes of the complement-mediated antibody-dependent enhancement of HIV-1 infection. AIDS. 1997;11(8):949–958. [PubMed] [Google Scholar]
- Qiao S., Jiang Z., Tian X., Wang R., Xing G., Wan B. Porcine FcgammaRIIb mediates enhancement of porcine reproductive and respiratory syndrome virus (PRRSV) infection. PLoS One. 2011;6(12) doi: 10.1371/journal.pone.0028721. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Quinlan B.D., Mou H., Zhang L., Guo Y., He W., Ojha A. The SARS-CoV-2 receptor-binding domain elicits a potent neutralizing response without antibody-dependent enhancement. bioRxiv. 2020 doi: 10.1101/2020.04.10.036418. 2020.2004.2010.036418. [CrossRef] [Google Scholar]
- Randow F., Macmicking J.D., James L.C. Cellular self-defense: How cell-autonomous immunity protects against pathogens. Science. 2013;340(6133):701–706. [PMC free article] [PubMed] [Google Scholar]
- Rathore A.P.S., St John A.L. Cross-reactive immunity among flaviviruses. Frontiers in Immunology. 2020;11:334. doi: 10.3389/fimmu.2020.00334. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Robinson W.E., Montefiori D.C., Mitchell W.M. Complement-mediated antibody-dependent enhancement of HIV-1 infection requires CD4 and complement receptors. Virology. 1990;175(2):600–604. [PubMed] [Google Scholar]
- Rodrigo W.W., Jin X., Blackley S.D., Rose R.C., Schlesinger J.J. Differential enhancement of dengue virus immune complex infectivity mediated by signaling-competent and signaling-incompetent human Fcgamma RIA (CD64) or FcgammaRIIA (CD32) Journal of Virology. 2006;80(20):10128–10138. doi: 10.1128/JVI.00792-06. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Saura M., Zaragoza C.A., Quick R., Hohenadl C., Lowenstein J., Lowenstein C. An antiviral mechanism of nitric oxide: Inhibition of a viral protease. Immunity. 1999;10(1):21–28. [PMC free article] [PubMed] [Google Scholar]
- Sautter C.A., Trus I., Nauwynck H., Summerfield A. No evidence for a role for antibodies during vaccination-induced enhancement of porcine reproductive and respiratory syndrome. Viruses. 2019;11(9) doi: 10.3390/v11090829. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Savidis G., Mcdougall W.M., Meraner P., Perreira J.M., Portmann J.M., Trincucci G. Identification of zika virus and dengue virus dependency factors using functional genomics. Cell Reports. 2016;16(1):232–246. [PubMed] [Google Scholar]
- Schlesinger J.J., Chapman S.E. Influence of the human high-affinity IgG receptor FcγRI (CD64) on residual infectivity of neutralized dengue virus. Virology. 1999;260(1):84–88. [PubMed] [Google Scholar]
- Schweitzer B.K., Chapman N.M., Iwen P.C. Overview of the flaviviridae with an emphasis on the japanese encephalitis group viruses. Laboratory Medicine. 2009;40(8):493–499. doi: 10.1309/lm5yws85njpcwesw. [CrossRef] [Google Scholar]
- Screaton G., Mongkolsapaya J., Yacoub S., Roberts C. New insights into the immunopathology and control of dengue virus infection. Nature Reviews. Immunology. 2015;15(12):745–759. doi: 10.1038/nri3916. [PubMed] [CrossRef] [Google Scholar]
- Sharma A. It is too soon to attribute ADE to COVID-19. Microbes and Infection. 2020;22(4-5):158. doi: 10.1016/j.micinf.2020.03.005. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Shi P., Su Y., Li Y., Zhang L., Lu D., Li R. The alternatively spliced porcine FcgammaRI regulated PRRSV-ADE infection and proinflammatory cytokine production. Developmental and Comparative Immunology. 2019;90:186–198. doi: 10.1016/j.dci.2018.09.019. [PubMed] [CrossRef] [Google Scholar]
- Shi P., Zhang L., Wang J., Lu D., Li Y., Ren J. Porcine FcepsilonRI mediates porcine reproductive and respiratory syndrome virus multiplication and regulates the inflammatory reaction. Virologica Sinica. 2018;33(3):249–260. doi: 10.1007/s12250-018-0032-3. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Shukla R., Shanmugam R.K., Ramasamy V., Arora U., Batra G., Acklin J.A. Zika virus envelope nanoparticle antibodies protect mice without risk of disease enhancement. eBioMedicine. 2020;54:102738. doi: 10.1016/j.ebiom.2020.102738. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Slon-Campos J.L., Dejnirattisai W., Jagger B.W., Lopez-Camacho C., Wongwiwat W., Durnell L.A. A protective Zika virus E-dimer-based subunit vaccine engineered to abrogate antibody-dependent enhancement of dengue infection. Nature Immunology. 2019;20(10):1291–1298. doi: 10.1038/s41590-019-0477-z. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Sridhar S., Luedtke A., Langevin E., Zhu M., Bonaparte M., Machabert T. Effect of dengue serostatus on dengue vaccine safety and efficacy. The New England Journal of Medicine. 2018;379(4):327–340. doi: 10.1056/NEJMoa1800820. [PubMed] [CrossRef] [Google Scholar]
- Sun H., Chen Q., Lai H. Development of antibody therapeutics against flaviviruses. International Journal of Molecular Sciences. 2017;19(1) doi: 10.3390/ijms19010054. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Takada A., Ebihara H., Feldmann H., Geisbert T.W., Kawaoka Y. Epitopes required for antibody-dependent enhancement of Ebola virus infection. The Journal of Infectious Diseases. 2007;196(Suppl 2):S347–S356. doi: 10.1086/520581. [PubMed] [CrossRef] [Google Scholar]
- Takada A., Feldmann H., Ksiazek T.G., Kawaoka Y. Antibody-dependent enhancement of Ebola virus infection. Journal of Virology. 2003;77(13):7539–7544. doi: 10.1128/jvi.77.13.7539-7544.2003. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Tang B., Xiao Y., Sander B., Kulkarni M.A., Radam-Lac Research T., Wu J. Modelling the impact of antibody-dependent enhancement on disease severity of Zika virus and dengue virus sequential and co-infection. Royal Society Open Science. 2020;7(4):191749. doi: 10.1098/rsos.191749. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Taylor A., Foo S.S., Bruzzone R., Vu Dinh L., King N.J.C., Mahalingam S. Fc receptors in antibody-dependent enhancement of viral infections. Immunological Reviews. 2015;268(1):340–364. [PMC free article] [PubMed] [Google Scholar]
- Tetro J.A. Is COVID-19 receiving ADE from other coronaviruses? Microbes and Infection. 2020;22(2):72–73. doi: 10.1016/j.micinf.2020.02.006. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Thein T.L., Wong J., Leo Y.S., Ooi E.E., Lye D., Yeo T.W. Association between increased vascular nitric oxide bioavailability and progression to dengue hemorrhagic fever in adults. The Journal of Infectious Diseases. 2015;212(5):711–714. doi: 10.1093/infdis/jiv122. [PubMed] [CrossRef] [Google Scholar]
- Thieblemont N. CR1 (CD35) and CR3 (CD11b/CD18) mediate infection of human monocytes and monocytic cell lines with complement-opsonized HIV independently of CD4. Clinical and Experimental Immunology. 2010;92(1):106–113. doi: 10.1111/j.1365-2249.1993.tb05955.x. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Tsai T.T., Chuang Y.J., Lin Y.S., Chang C.P., Wan S.W., Lin S.H. Antibody-dependent enhancement infection facilitates dengue virus-regulated signaling of IL-10 production in monocytes. PLoS Neglected Tropical Diseases. 2014;8(11) doi: 10.1371/journal.pntd.0003320. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Ubol S., Halstead S.B. How innate immune mechanisms contribute to antibody-enhanced viral infections. Clinical and Vaccine Immunology. 2010;17(12):1829–1835. doi: 10.1128/CVI.00316-10. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Ubol S., Phuklia W., Kalayanarooj S. Mechanisms of immune evasion induced by a complex of dengue virus and preexisting enhancing antibodies. Journal of Infectious Diseases. 2010;201(6):923–935. [PubMed] [Google Scholar]
- Viktorovskaya O.V., Greco T.M., Cristea I.M., Thompson S.R. Identification of RNA binding proteins associated with dengue virus RNA in infected cells reveals temporally distinct host factor requirements. PLoS Neglected Tropical Diseases. 2016;10(8) doi: 10.1371/journal.pntd.0004921. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- von Kietzell K., Pozzuto T., Heilbronn R., Grossl T., Fechner H., Weger S. Antibody-mediated enhancement of parvovirus B19 uptake into endothelial cells mediated by a receptor for complement factor C1q. Journal of Virology. 2014;88(14):8102–8115. doi: 10.1128/JVI.00649-14. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Walls A.C., Xiong X., Park Y.J., Tortorici M.A., Snijder J., Quispe J. Unexpected receptor functional mimicry elucidates activation of coronavirus fusion. Cell. 2019;176(5):1026–1039. doi: 10.1016/j.cell.2018.12.028. e1015. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Wan B., Chen X., Li Y., Pang M., Bao D. Porcine FcγRIIb mediated PRRSV ADE infection through inhibiting IFN-β by cytoplasmic inhibitory signal transduction. International Journal of Biological Macromolecules. 2019;138 [PubMed] [Google Scholar]
- Wan Y., Shang J., Sun S., Tai W., Chen J., Geng Q. Molecular mechanism for antibody-dependent enhancement of coronavirus entry. Journal of Virology. 2020;94(5) doi: 10.1128/JVI.02015-19. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Wang T.T., Sewatanon J., Memoli M.J., Wrammert J., Bournazos S., Bhaumik S.K. IgG antibodies to dengue enhanced for Fc gamma RIIIA binding determine disease severity. Science. 2017;355(6323):395–398. [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Si L.L., Guo X.L., Cui G.H., Fang D.Y., Zhou J.M. Substitution of the precursor peptide prevents anti-prM antibody-mediated antibody-dependent enhancement of dengue virus infection. Virus Research. 2017;229:57–64. doi: 10.1016/j.virusres.2016.12.003. [PubMed] [CrossRef] [Google Scholar]
- Wang Y., Si L., Luo Y., Guo X., Zhou J., Fang D. Replacement of pr gene with Japanese encephalitis virus pr using reverse genetics reduces antibody-dependent enhancement of dengue virus 2 infection. Applied Microbiology and Biotechnology. 2015;99(22):9685–9698. doi: 10.1007/s00253-015-6819-3. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Wang S.F., Tseng S.P., Yen C.H., Yang J.Y., Tsao C.H., Shen C.W. Antibody-dependent SARS coronavirus infection is mediated by antibodies against spike proteins. Biochemical and Biophysical Research Communications. 2014;451(2):208–214. doi: 10.1016/j.bbrc.2014.07.090. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Wang J., Zand M.S. The potential for antibody-dependent enhancement of SARS-CoV-2 infection: Translational implications for vaccine development. Journal of Clinical and Translational Science. 2020;5(1):1–4. doi: 10.1017/cts.2020.39. [CrossRef] [Google Scholar]
- Wang M., Yang F., Huang D., Huang Y., Zhang X., Wang C. Anti-Idiotypic antibodies specific to prM monoantibody prevent antibody dependent enhancement of dengue virus infection. Frontiers in Cellular and Infection Microbiology. 2017;7:157. doi: 10.3389/fcimb.2017.00157. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Wang G., Yu Y., Cai X., Zhou E.-M., Zimmerman J.J. Effects of PRRSV infection on the porcine thymus. Trends in Microbiology. 2020;28(3):212–223. [PubMed] [Google Scholar]
- Wang Q., Zhang L., Kuwahara K., Li L., Liu Z., Li T. Immunodominant SARS coronavirus epitopes in humans elicited both enhancing and neutralizing effects on infection in non-human primates. ACS Infectious Diseases. 2016;2(5):361–376. doi: 10.1021/acsinfecdis.6b00006. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Weaver S.C., Barrett A.D. Transmission cycles, host range, evolution and emergence of arboviral disease. Nature Reviews. Microbiology. 2004;2(10):789–801. doi: 10.1038/nrmicro1006. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Wei C.J., Crank M.C., Shiver J., Graham B.S., Mascola J.R., Nabel G.J. Next-generation influenza vaccines: Opportunities and challenges. Nature Reviews. Drug Discovery. 2020;19(4):239–252. doi: 10.1038/s41573-019-0056-x. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Widjaja I., Wang C., van Haperen R., Gutierrez-Alvarez J., van Dieren B., Okba N.M.A. Towards a solution to MERS: protective human monoclonal antibodies targeting different domains and functions of the MERS-coronavirus spike glycoprotein. Emerging Microbes & Infections. 2019;8(1):516–530. doi: 10.1080/22221751.2019.1597644. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Wrapp D., De Vlieger D., Corbett K.S., Torres G.M., Wang N., Van Breedam W. Structural basis for potent neutralization of betacoronaviruses by single-domain camelid antibodies. Cell. 2020;181(5):1004–1015. doi: 10.1016/j.cell.2020.04.031. e1015. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Yoon K.J., Wu L.L., Zimmerman J.J., Hill H.T., Platt K.B. Antibody-dependent enhancement (ADE) of porcine reproductive and respiratory syndrome virus (PRRSV) infection in pigs. Viral Immunology. 1996;9(1):51–63. [PubMed] [Google Scholar]
- Zhang L., Chen J., Wang D., Li N., Qin Y., Du D. Ligation of porcine Fc gamma receptor III inhibits levels of antiviral cytokine in response to PRRSV infection in vitro. Research in Veterinary Science. 2016;105:47–52. doi: 10.1016/j.rvsc.2016.01.009. [PubMed] [CrossRef] [Google Scholar]
- Zhang A., Duan H., Li N., Zhao L., Xiao S. Heme oxygenase-1 metabolite biliverdin, not iron, inhibits porcine reproductive and respiratory syndrome virus replication. Free Radical Biology & Medicine. 2016;102:149–161. [PubMed] [Google Scholar]
- Zhang L., Li W., Sun Y., Kong L., Xu P., Xia P. Antibody-mediated porcine reproductive and respiratory syndrome virus infection downregulates the production of interferon-α and tumor necrosis factor-α in porcine alveolar macrophages via Fc gamma receptor I and III. Viruses. 2020;12(2) [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Shen H., Gong Y., Pang X., Yi M., Guo L. Development of a dual-functional conjugate of antigenic peptide and Fc-III mimetics (DCAF) for targeted antibody blocking. Chemical Science. 2019;10(11):3271–3280. doi: 10.1039/c8sc05273e. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Zhou Y., Liu Z., Li S., Xu W., Zhang Q., Silva I.T. Enhancement versus neutralization by SARS-CoV-2 antibodies from a convalescent donor associates with distinct epitopes on the RBD. Cell Reports. 2021;34(5):108699. doi: 10.1016/j.celrep.2021.108699. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [PMC free article] [PubMed] [CrossRef] [Google Scholar]