Authors: By Angela Betsaida B. Laguipo, BSN
In an effort to stem the spread of the coronavirus disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pathogen, vaccination efforts have been implemented in most countries.
Two of the vaccines developed to prevent SARS-CoV-2 infection are the novel messenger ribonucleic acid (mRNA) vaccines: the Pfizer-BioNTech (BNT162b2) and the Moderna (mRNA-1273) vaccines.
Despite this vaccine type’s novelty, mRNA vaccines have been studied by scientists for decades. Moreover, both vaccines underwent extensive testing and rigorous clinical trials to determine their safety and efficacy.
Against the backdrop of a global public health emergency, clinical trials and regulatory body approval for both mRNA vaccines had been compressed into a shorter timespan than usual for novel pharmaceuticals like these. However, no safety measures had been compromised in the process, and all of the usual protocols were still followed within this expedited timeframe.
The pharmaceutical companies made this possible by conducting overlapping clinical trials, which involve human testing and occur in three phases that scale up each time. Meanwhile, some regulatory bodies – like the Medicines and Healthcare products Regulatory Agency (MHRA) in the UK – conducted a ‘rolling review’ of the trial data for both vaccines as and when it was made available. This is an emergency measure that is employed to speed up the process in pressing instances like global pandemics.
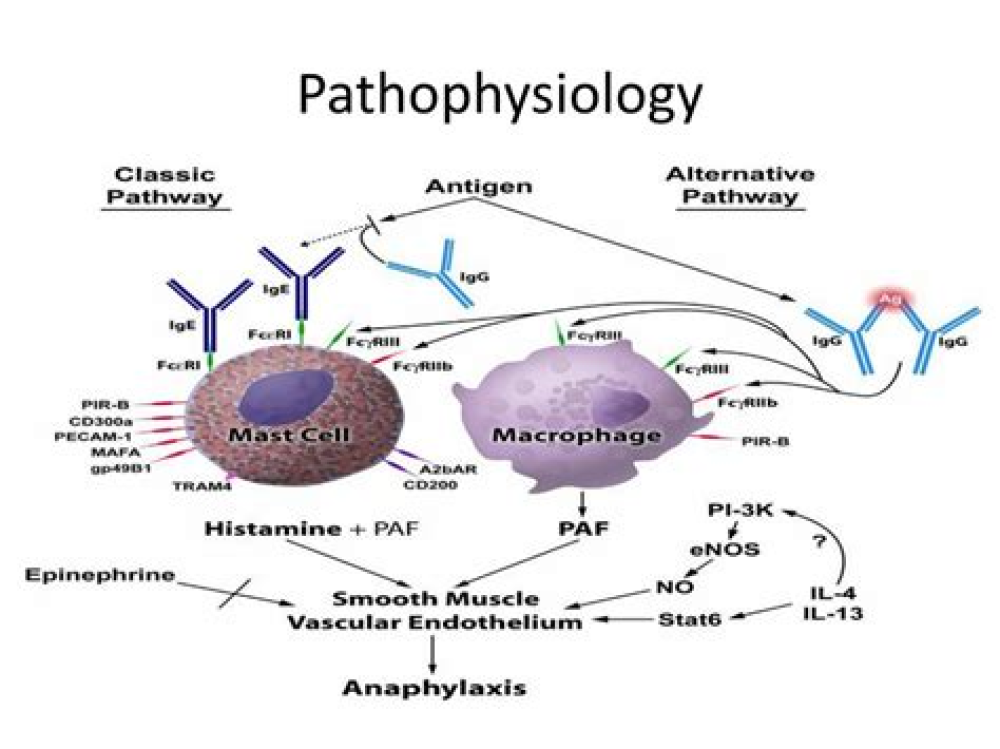
This said, as with all pharmaceuticals, a small subset of individuals are unfortunately susceptible to adverse reactions, something which is not always detected at the clinical trial level. This includes, though is not limited to, anaphylaxis or allergic reactions to ingredients that vaccines sometimes contain or are stored in.
A new report from a team of scientists at the Centre for Research in Molecular Modeling, Department of Chemistry and Biochemistry, Concordia University in Canada, has aimed to determine the potential causes of anaphylaxis or allergic reactions reported in some individuals after receiving COVID-19 vaccines, including the mRNA vaccines by Pfizer-BioNTech and Moderna.
For More Information: https://www.news-medical.net/news/20210601/Scientists-explain-possible-causes-of-anaphylaxis-following-mRNA-COVID-19-vaccination.aspx