Author links open overlay panelChristophMeier123A. RaduAricescu13ReneAssenberg2Robin T.Aplin2Robert J.C.Gilbert13Jonathan M.Grimes123David I.Stuart123
Summary
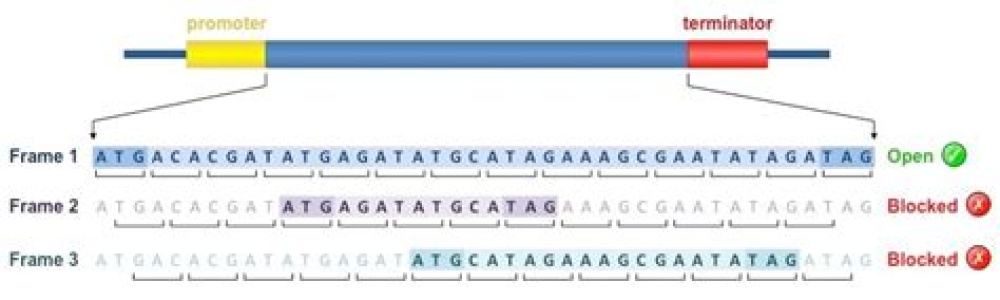
To achieve the greatest output from their limited genomes, viruses frequently make use of alternative open reading frames, in which translation is initiated from a start codon within an existing gene and, being out of frame, gives rise to a distinct protein product. These alternative protein products are, as yet, poorly characterized structurally. Here we report the crystal structure of ORF-9b, an alternative open reading frame within the nucleocapsid (N) gene from the SARS coronavirus. The protein has a novel fold, a dimeric tent-like β structure with an amphipathic surface, and a central hydrophobic cavity that binds lipid molecules. This cavity is likely to be involved in membrane attachment and, in mammalian cells, ORF-9b associates with intracellular vesicles, consistent with a role in the assembly of the virion. Analysis of ORF-9b and other overlapping genes suggests that they provide snapshots of the early evolution of novel protein folds.
For More Information: https://www.sciencedirect.com/science/article/pii/S0969212606002516